Translational Immuno-Pharmacology
Research
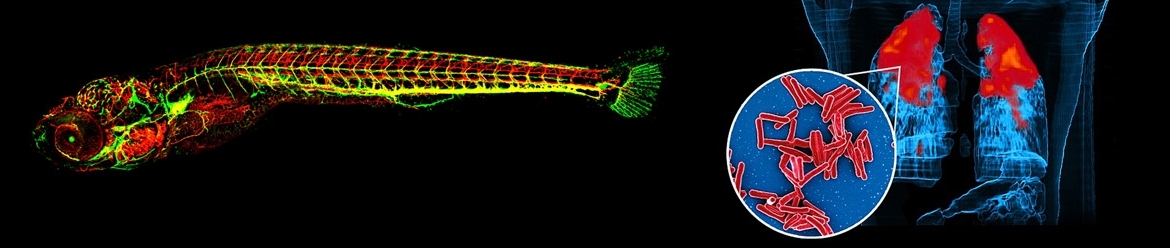
Tuberculosis causes 1.5 million deaths yearly and anti-tuberculosis therapies are threatened by emergence of drug resistance. Development of innovative drug combinations should be accelerated with the use of translational pharmacological models. Moreover, host-directed therapies (HDT), which stimulate the immune system to clear the infection, are suggested as complementary strategy to antibiotics, to improve treatment outcomes and shorten treatment duration. Despite the potential of HDTs against tuberculosis, no clinically effective HDTs against tuberculosis are available due to translational challenges related to differences in the immunopharmacological effects of HDTs between preclinical species and patients.
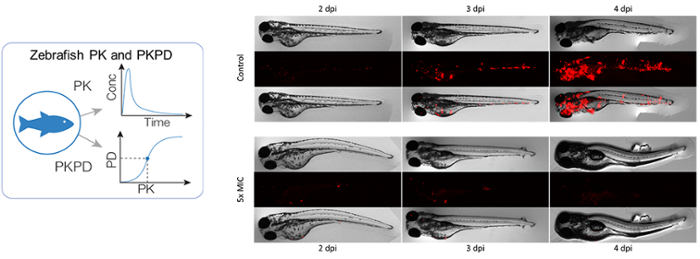
In addition to existing murine disease models, the zebrafish larva is an attractive model organism to study infectious disease and their interplay with the immune system and therapies, earlier in drug development. A TB disease model has been established based on Mycobacterium marinum infection through different routes, with serial fluorescence microscopy observations of both bacteria and immune cells to study their response to infection and treatment. Proof-of-concept of translatability of pharmacokinetics-pharmacodynamic findings from zebrafish larvae to higher vertebrates has been shown for anti-TB drugs such as isoniazid [Van Wijk et al, BJP 177(24), 2020].
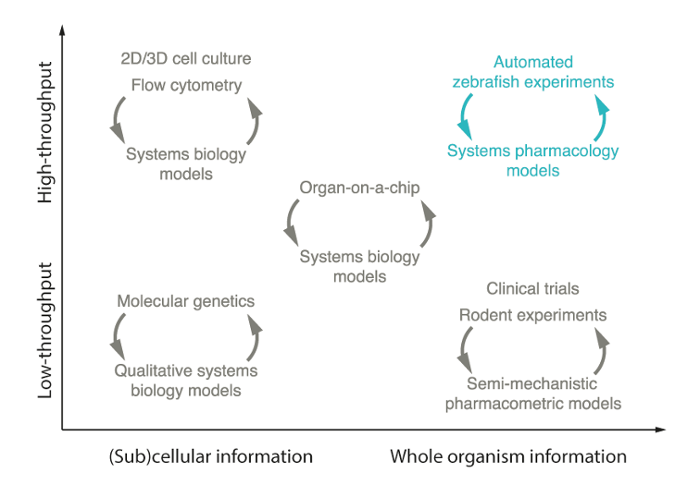
The Translational Immuno-Pharmacology group excels in both experimental and computational innovation, and close collaboration between experimentalists and computational modellers follows the Team Science approach.
Experimentally, the group consolidates the translational pharmacokinetic-pharmacodynamic experiments, including exposure-time experiments in whole-organism and nanoscale blood sampling, and response experiments with fluorescence microscopy. HDT and their interaction with pathogen and treatment are studied and quantified through biomarker analysis, such as cytokines, immune cells, genetic, or (fluorescence) microscopy.
Computationally, models incorporating pharmacokinetics, pharmacodynamics, and immunology are developed to quantify both antibiotic and host-directed drug effects in preclinical species (e.g. mice, zebrafish) and predict their efficacy in patients. Different modelling techniques such as population and semi-mechanistic models, agent-based models, and systems (immuno)pharmacology are employed.
Research projects
- Translating anti-tuberculosis drug efficacy from zebrafish to higher vertebrates
- Translational immune-pharmacology of host-directed therapy against tuberculosis
- Host-directed therapy against meticilline-resistant Staphylococcus aureus (MRSA)
- Optimizing combination treatment of antibiotic and host-directed therapy against tuberculosis