Leiden Early Drug Discovery & Development
More effective blocking of CCR2 receptor
The discovery of new medicines is a tedious and lengthy process. On average, over 10,000 molecules need to be studied for one to become a drug and reach the patient. Part of that process are the very costly clinical trials in humans, and candidate drugs often fail due to side effects or lack of efficacy. Apparently, to predict a drug’s performance in man, the traditional pharmacological parameters such as “affinity” and ”selectivity”, are insufficient.
Improving how drugs bind to their targets
To improve this situation, we have developed novel compounds and cellular assays to proof the value of novel drug discovery concepts, such as ‘Allosteric Modulation’ and ‘Drug-Target binding kinetics.’
Since (in retrospect) it was shown that many drugs are effective because they bind to their target for a long time, improving a compound’s target residence time could have great clinical value. In this respect, we were the first to show that the binding of a drug to its target can be tuned prospectively using medicinal chemistry.
Blocking a receptor from two sides
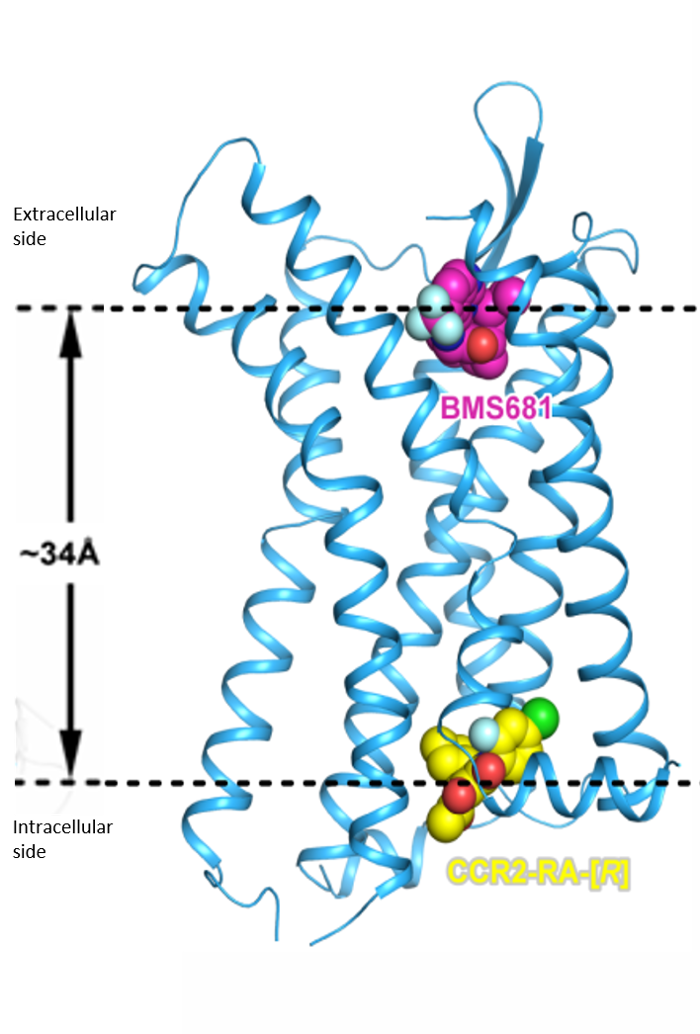
To be able to study new medicines, it’s key to have a sense of how molecules in the cell work, and how compounds can bind to proteins targets to modulate their function. In that sense, we have done great work on the CCR2 receptor, a molecule that is present in cell walls.
We were the first to show the structure of CCR2, and that the protein can be inhibited from inside the cell by a small molecule. Specifically, X-ray crystallography showed that two molecules can simultaneously bind the receptor, turning it “off” by different but mutually reinforcing mechanisms. One of the small molecules binds the outside face of the receptor (on the already known orthosteric site) and thereby directly blocks binding of the natural chemokines that normally turn the receptor “on.” The other small molecule binds the face of the receptor inside the cell, where the G-protein normally binds, preventing inflammatory signal transmission.
Preventing inflammation through long-term binding
The latter binding site had at that time never been seen before. Importantly, inhibition of the chemokine receptor via this allosteric site either with non-covalent or covalent inhibitors results in so-called insurmountable antagonism. Note that the latter can also be obtained by using competitive/orthosteric antagonists with long residence time.
This concept of insurmountable antagonism has great potential for combating diseases with an inflammatory component, i.e. when often high (local) concentrations of the endogenous ligand are present, such as chemokines. It goes without saying, that the work presented on CCR2 is representative for other GPCRs as well.