Quantitative Clinical Pharmacology
Research
Knowledge on how to adjust a drug dose in special patient populations such as (prematurely born) neonates or children, obese individuals or critically-ill patients, is not only crucial for novel compounds, but also for existing drugs which are often used in an off-label manner in these special patient groups.
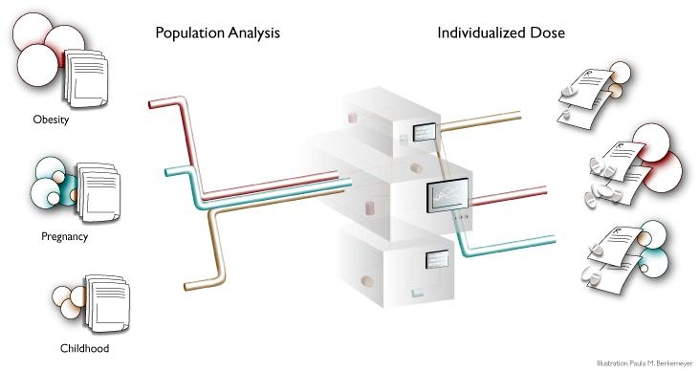
Within the context of close collaborations with our clinical partners, we gather pharmacokinetic (PK) and pharmacodynamic (PD) data in these special patient populations through clinical studies, routine clinical practice or a combination of both (e.g. by measurements in scavenged samples samples). Pharmacokinetic (PK) data relate to concentrations of the drug of interest while pharmacodynamic (PD) data relate to the effect of the drug in the patient. These clinical PK and PD data are collected in (prematurely born) neonates, children, morbidly obese patients, obese adolescents, or critically ill patients.
Using advanced statistical techniques that are applied to these clinical PK and PD data, predictors of variability (i.e. covariates) are identified that can subsequently be used to guide dosing in these groups. As covariates, for instance bodyweight, severity of illness, age, or a biomarker can be identified. Advanced statistical techniques such as the population approach are not only crucial to identify covariates but also to handle sparse data sets from neonates and children, as in those cases only limited clinical observations can be obtained because of small patient numbers and restricted blood sampling possibilities in children. With the use of these so-called population pharmacokinetic and pharmacodynamic (PK-PD) analyses applied to clinical data, individualized dosing guidelines have been derived for many different drugs in different special patient populations.
As it would require both a large burden to individual children and tremendous budgets to study all drugs across the entire pediatric age range in clinical trials, we also aim to develop system pediatric models in which we integrate physiological concepts with drug-related information. The purpose of these models is to provide general insight into how to predict dosages for different types of drugs in children of different ages. The use of physiologically-based methods within this approach is crucial as it allows to predict how drug action may change in case of changes in the human system as a result of ageing (system specific properties) taking into account the specific properties of the drug (drug specific properties).
Ultimately, these efforts should lead to quantitative clinical pharmacology models that can not only predict the efficacy and safety of the drug studied, but also of other (related) drugs in each of these special patient populations.

