Medical Systems Biophysics and Bioengineering
Research lines
Physics and Engineering Approaches to Biomedicine and Pharmacology
The Mashaghi lab at LACDR is developing the following three research lines:
1. Single-molecule biophysics and molecular topology
Folding pathways are traditionally studied for proteins in isolation, even though chaperones and other interacting partners critically interfere with folding and assembly of biomolecules in vivo in constructive or destructive ways. Consequently, our understanding of folding processes in life is fundamentally incomplete. Our group addresses this question with a single-molecule approach and modeling techniques.
1.1 Single-molecule force spectroscopy
Conventional biochemical assays involve millions of billions of molecules often reacting within an aqueous environment. The measured molecular properties are then summed or averaged over an ensemble of molecules as described in statistical thermodynamics. This leads to a high signal-to-noise ratio but also to information loss. Particularly in protein folding, transient intermediate molecular states are hidden in bulk measurements because the proteins are not synchronized.
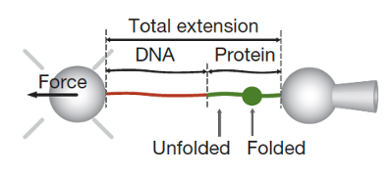
Within the last few decades, it has become possible not only to detect individual molecules but also to perform measurements on single molecules. In single-molecule force spectroscopy, the protein under study can be perturbed mechanically, for example, by pulling the ends of the molecule. By monitoring the extension length as a function of force, intermediate conformations in the unfolding and folding pathways can be visualized.
In addition, after mechanical unfolding of the protein, the refolding pathway can be investigated by relaxing the applied force and monitoring the extent of refolding as a function of refolding time. Forces and distances can be monitored in real time with piconewton and nanometer resolution.
1.2 Circuit topology
Folded biomolecules display a bewildering structural complexity and diversity. They have therefore been analyzed in terms of generic topological features. For instance, folded proteins may be knotted, have beta-strands arranged into a Greek-key motif, or display high contact order.
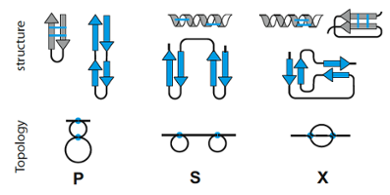
Our group is developing a method to formally describe the topology of all folded linear chains and hence provide a general classification and analysis framework for a range of biomolecules. We apply this circuit topology notion to study the equivalence of folded chains, the engineering of artificial RNA structures and DNA origami, the topological structure of genomes, and the implications of topology for protein folding.
2. Medical systems biophysics and pharmacophysics
Our group develops tools to analyse topology of interactions in complex systems (e.g. signalling network, cell-to-cell communication networks, and human disease networks). We hope to demonstrate how these topology analyses help solving biomedical problems such as medical diagnostic and pharmacological problems.
2.1 Medical diagnostic
We are exploring the use of statistical physics for medical diagnostics. We use omics data to generate symptom-based human disease network and to investigate the connection between clinical manifestations of diseases and their underlying molecular mechanisms. Our analysis would potentially help not only in understanding disease etiology and progression dynamics, but also in improving current diagnostic strategies. In particular, we are aiming at (i) developing consensus diagnostics flowcharts and (ii) improving the efficiency of diagnostic approaches by including the often-ignored disease-disease correlations.
2.2 Signaling network
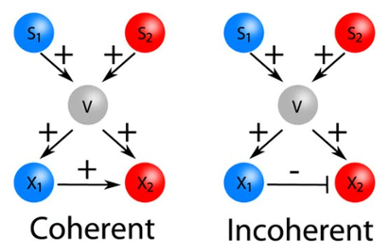
Structural analysis of biochemical networks helps in target selection thereby improving effectiveness and toxicity of drug treatment strategies. A significant step forward in rationalizing the choice of drug targets was realized following advances in molecular biology and network theory.
In recent years, many components of signaling pathways and their interactions are discovered and represented using detailed knowledge of the molecular connections within the signaling network. These developments allow for selecting targets on a stronger rational basis.
The Mashaghi lab is interested in studying signaling network dynamics and topology in order to reveal constraints on the form of the pharmacological response and to provide insights into toxicity and effectiveness of drug treatment strategies.
2.3 Cell-to-cell communications
Our body organs typically function through multicellular communication. Deep understanding of organs’ function and dysfunction requires studying the underlying cell-cell interaction networks. The Mashaghi lab is focused on understanding communications between immune cells and their targets. We are interested in finding how those communications shape the disease phenotypes and response to therapies.
Our group develop theoretical models based on data from in vitro as well as animal studies (conducted by us or our collaborators), to understand cellular interactions in cancer, autoimmunity and infection. The aim is to provide mechanistic insights into inflammatory diseases and to develop optimal strategies for pharmaco- and immuno-therapy.

3. Disease models-on-chips and immunoengineering
3.1 In vitro model development
We develop organ-on-chips to model the interactions between immune systems and human organs and tissues and apply the models to understand the crosstalk between metabolism and immune signalling. In particular, we are interested in using these platforms to screen for inter-individual differences in disease progression and responses to therapy. We combine our previous expertise in surface sensing, chip fabrication, immunology and metabolomics to contribute to the nascent fields of immunoengineering and immunophysics. In the context of this project, the Mashaghi group collaborates with several industrial partners including MIMETAS (NL) and Agios Pharmaceuticals (USA).
3.2 Chemical and mechanical characterization of single-cells
In parallel, we are developing platforms for high-resolution phenotyping of single human cells. This research aims at gaining insights into the origin of the inter-individual variations and its potential links to stochasticity in cellular signalling. The stochastic nature of gene expression in human cells is increasingly understood, but how it impacts the immune response remains unclear.

We focus on blood cells including immune cells and cancer cells and will characterize them using mass cytometric imaging, single-cell metabolic analysis (both using fluorescence and label-free approaches), and single-cell mechanical micromanipulation.