Molecular Physiology
Research
The current projects of the Molecular Physiology group focus on proteins of the endocannabinoid system, kinases and antibacterial targets. MSc- and BSc-students can contact Jessica van Krimpen-Kraaijenoord to apply for research internships.
Detection and modulation of endocannabinoid biosynthesis (Prof. Mario van der Stelt)
Endocannabinoids are endogenous signaling lipids that activate cannabinoid CB1 and CB2 receptors, which are also targeted by Δ9-tetrahydrocannabinol (THC), the main active ingredient in marijuana. Endocannabinoids are key regulators of neurotransmission in the brain. They participate in adaptive and maladaptive responses of diverse synapses via mechanisms involving multiple signaling cascades, in both presynaptic and postsynaptic compartments. Despite decades of research and technological advances accelerating the understanding of its contribution to brain function, our knowledge is yet incomplete. Because of the expanding legalization and liberalization policies regarding cannabis use, it is paramount to deepen our appreciation of the neurobiological mechanisms modulated by the endocannabinoid system and how it is affected by cannabis constituents.
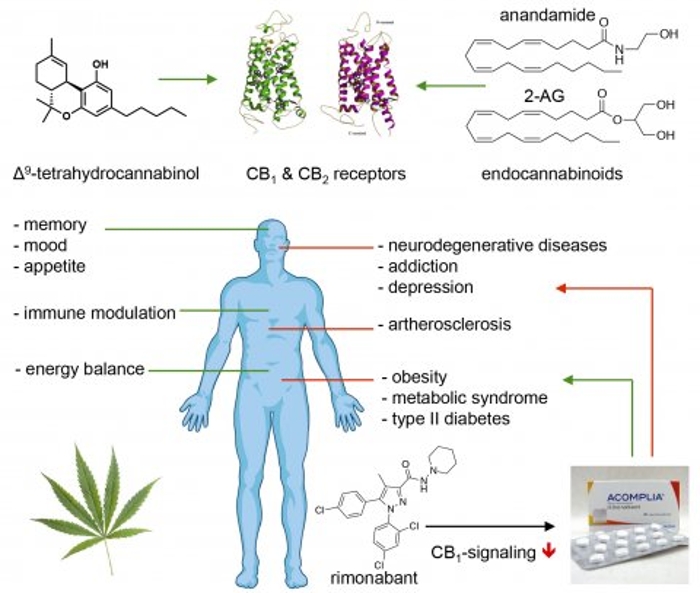
Important challenges include: why do we have multiple endocannabinoids? Is there any cell type or regional specificity of their contributions? Is there a bias for one of the two endocannabinoids to be mobilized: when, where and why? Is there an endocannabinoid transporter? How does aberrant endocannabinoid signaling lead to disease? Does medicinal and recreational cannabis use lead to therapeutic benefit or to neuropsychiatric disorders? How does cannabidiol act? Finally, arguably equally important, what are the emerging drugs targeting the endocannabinoid system?
In this research programme we develop assays, (fluorescent) ligands, mass spectrometry methods and activity-based probes to regulate and determine the activity of many of proteins of the endocannabinoid system (MAGL, DAGLα/β, AHBD6, ABHD12, FAAH, NAPE-PLD, CB1R and CB2R). We use different lead finding strategies, including high throughput screening and structure-based drug design, to identify and optimize molecules to control and visualize the activity of the proteins in preclinical disease models and human brain tissue.

Development of selective kinase inhibitors to treat cancer (Prof. Mario van der Stelt)
As part of Oncode Institute and in collaboration with several partners (e.g. Netherlands Translational Research Center, Leiden University Medical Center, Netherlands Cancer Institute, Hubrecht Institute and Pivot Park Screening Center) we have identified and profiled several novel kinase inhibitors that potentially can be used for the treatment of triple-negative breast cancer and acute myeloid leukemia. The goal of these projects is to optimize our leads into drug candidates. As part of this research line, activity-based protein profiling is being applied to the family of protein kinases to study inhibitor activity and selectivity in live cells. Furthermore, we use chemical proteomics to study the active kinome in various biological processes relevant to cancer.
Development of covalently reactive molecules as novel antibiotics (Dr. Stephan Hacker)
Antibiotic-resistant bacteria are a huge threat to human health. One main reason for efficient bacterial resistance development is, that all marketed antibiotics address a rather narrow scope of molecular targets. In our research, we profile bacterial proteomes for their interaction with covalent inhibitors and, in this way, identify new target proteins for antibiotics. In order to do so, we apply established reactive groups for covalent inhibitors that target cysteines and develop new chemotypes that address other amino acids with high selectivity. Using these compound libraries in phenotypic screening approaches, we identify antibiotically active compounds in the most relevant human pathogens. We, furthermore, develop tailored chemoproteomic methods that allow us to identify the targets of our hit compounds with resolution of the addressed amino acid residue proteome-wide. In this way, we deliver global maps of ligandable residues in the bacterial proteome that will be starting points for the development of antibiotics with entirely new modes-of-action.
Development and application of computational and artificial intelligence methods in early drug discovery projects (Dr. Anthe Janssen)
In close collaboration with Prof. Dr. Gerard van Westen at the Leiden Academic Centre for Drug Research (LACDR) state-of-the-art machine learning approaches are developed and applied to the ongoing drug research programs in the group. Language-based molecular generators and transformer-based computer vision models are employed to efficiently and accurately cover the vast drug-protein-interaction search space. More traditional techniques such as (high-throughput) docking and molecular dynamics studies are used to visualize drug-target interactions and improve our molecular designs.
Chemical-genetic tools for drug target validation (Dr. Tom van der Wel)
Target validation is an essential step in drug discovery and demonstrates that modulation of the target protein leads to a therapeutic benefit. We use chemical genetics – combining the acute mode-of-action of chemical probes and the specificity of genetics – to develop new methods for target validation. As part of this research, we generate tools to measure drug-target interactions and ligand selectivity, using rationally designed mutations that sensitize or abolish ligand binding to the target protein. Gene editing techniques (CRISPR/Cas9, base editors) are employed to introduce these mutations endogenously in relevant cell models. Furthermore, genetic screening methods are employed to identify functionally important and ligandable amino acids in disease-relevant proteins. In this way, we aim to find starting points for the development of new drugs with an innovative mechanism of action.
Expanding the druggability of the immune system (Madeline Kavanagh)
Our lab’s interdisciplinary approach relies on a combination of chemistry, biological, analytical and computational methods. We synthesize novel small molecule probes and analogues of bioactive natural products, and map their binding interactions using a combination of chemical biology and proteomic techniques. We also rely on medicinal chemistry to optimize small molecule protein ligands into potent and selective chemical probes or drug leads. We rely on emerging genetic techniques (e.g., CRISPR), biochemical and cell-based assay to robustly characterize the functional impact of small molecule-protein interactions, and apply the novel compounds we develop in primary immune cells or disease-relevant biological systems. We leverage advances in protein structural biology, human genetics data, and computational techniques to develop hypotheses, and discover, novel druggable, and functional sites on proteins.