Research project
Role of Chemokine Gradient Sensing in Ewing Sarcoma Progression, Angiogenesis and Immune Targeting
What are the biological and biophysical mechanisms that control chemokine gradient sensing and migration of immune, endothelial, and tumour cells in tumour development?
- Duration
- 2011 - 2015
- Contact
- Ewa Snaar-Jagalska
- Funding
-
 NWO, TOP-GO
NWO, TOP-GO
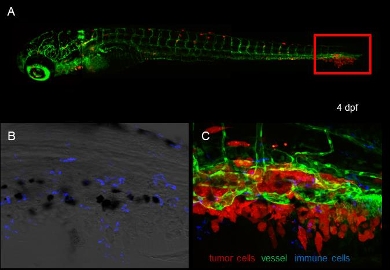
The interaction of Ewing sarcoma cells with the tumor microenvironment is essential for tumour growth, angiogenesis and metastasis. Chemokines secreted by tumour cells attract immune cells, particularly leukocytes to tumour sites to increase tumour growth, while other immune cells may enhance anti-tumour immunity. This integrative project at clinical, biological and biophysical level aims to generate fundamental knowledge of the chemokine network underlying Ewing sarcoma disease with potential application for novel anti-cancer treatments.
Ewing sarcoma is a malignant bone tumour most commonly found in children and young adults. Although the genetic abnormality causing Ewing sarcoma as consistent reciprocal chromosomal translocation is known, patients with metastases have very poor prognosis. The interaction of Ewing sarcoma cells with the tumour microenvironment is essential for tumour growth, angiogenesis and metastasis. The role of chemokines therein is ambivalent. In the current multidisciplinary project we want to further develop, explore, and experimentally test concepts and molecular components of chemokine gradient sensing that leads to migration of immune, endothelial and tumour cells during tumor progression and angiogenesis in Ewing sarcoma. The project aims at a molecular/mechanistic view of gradient sensing in tumour development. The fundamental knowledge generated in the course of the project has potential for application in anti-tumour immunity and anti-angiogenetic therapies for cancer treatment. Along with the level of complexity the current research program is subdivided into 3 themes that cover the range from the pathology to the characterization of the chemokine receptor molecule. Three PhD-students will be working in close collaboration to maintain coherence and secure knowledge transfer across disciplines. We will
- In theme 1 identify novel chemokines and receptors from EWS cells for interfering with cell migration and angiogenesis
- In theme 2 exploit a zebrafish xenotransplant model for in vivo validation of these chemokines, and characterize receptor functionality in angiogenic, immune and host responses
- In theme 3 elucidate the physical mechanism of chemokine recognition in single-cell migration assay with focus on the contribution of specific receptors, their mobility, organization in domains and the dynamic reorganization of the plasma membrane interactions on single-molecule stimulation ex vivo.
- Tulotta C, Stefanescu C, Torraca V, Meijer AM and Snaar Jagalska BE (2016) CXCR4 signaling in the tumor microenvironment orchestrates experimental metastasis formation by controlling myeloid cell motility and response to malignant cells. Submitted.
- Beletkaia E, Fenz SF, Pomp W, Snaar-Jagalska BE, Hogendoorn P, Schmidt T (2016) Insight on CXCR4 signaling control at the plasma membrane through endocytosis. BBA 1863:607-16.
- Tulotta C, Stefanescu C, Beletkaia E, Busmann J, Schmidt T and Snaar-Jagalska BE (2016) Inhibition of signaling between human CXCR4 and zebrafish ligands by the small molecule IT1t impairs the formation of triple-negative breast cancer early metastases in a zebrafish xenograft. Dis. Model Mech. 9: 141–153.
- Tulotta C, He S, Chen L, Groenewoud A, van der Ent W, Meijer AH, Spaink HP, Snaar-Jagalska BE (2016) Imaging of Human Cancer Cell Proliferation, Invasion, and Micrometastasis in a Zebrafish Xenogeneic Engraftment Model. Methods Mol Biol. 2016;1451:155-69. doi: 10.1007/978-1-4939-3771-4_11.
- van der Ent W, Veneman WJ, Groenewoud A, Chen L, Tulotta C, Hogendoorn PC, Spaink HP, Snaar-Jagalska BE (2016) Automation of Technology for Cancer Research. Adv Exp Med Biol. 2016;916:315-32. doi: 10.1007/978-3-319-30654-4_14. Review.
- Tulotta C, He S, van der Ent W, Chen L, Groenewoud A, Spaink HP, Snaar-Jagalska BE (2016) Imaging Cancer Angiogenesis and Metastasis in a Zebrafish Embryo Model. Adv Exp Med Biol. 2016;916:239-63. doi: 10.1007/978-3-319-30654-4_11. Review
- Evensen L, Johansen PL, Koster G, Zhu K, Herfindal L, Speth M, Hildahl J, Bagherifam S, Tulotta C, Fenaroli F, Prasmickaite L, Mælandsmo G, Snaar-Jagalska BE, Griffiths G (2016) Zebrafish as a model system for characterization of nanoparticles against cancer. Nanoscale. 8: 862-877.
- Sand LG, Scotlandi K, Berghuis D, Snaar-Jagalska BE, Picci P, Schmidt T, Szuhai K, Hogendoorn PC (2015) CXCL14, CXCR7 expression and CXCR4 splice variant ratio associate with survival and metastases in Ewing sarcoma patients. Eur. J. Cancer. 51: 2624-33.
