Drugging the undruggable: NWO Open Competition grant for Alireza Mashaghi
Finding structure in disordered proteins and developing drugs for undruggable diseases: it might sound like mission impossible, but pharmacologist Alireza Mashaghi and his team are right on top of it. Their project was awarded by NWO through the Open Competition Domain Science -XS, a competition that encourages curiosity-driven and bold research.
In order to do its job, a protein must fold into a specific shape. When designing drugs, pharmacologists make use of this principle. Drugs will fit the well-defined shape of this protein, and once they’re bound, they do their job: they interfere with the function of the sick-making protein. There’s only one problem with this approach: what if a protein is not structured?
‘Nearly one third of our proteins are disordered or contain a disordered region,’ says Mashaghi. ‘They have no structure and are highly dynamic. For these proteins, we need a fundamentally new approach. Most of our drugs are designed for the ordered parts, and for those rare drugs that target the disordered region, we have no idea of how the drug functions.’
No drugs to suppress disordered proteins
The disordered region of a protein often drives complex interaction with other cellular proteins and is often very important. Because of that, they can be involved in different types of diseases, such as muscular dystrophy and different types of cancer. Mashaghi: ‘Take the Androgen hormone receptor, in which more than half of the protein is disordered This receptor protein is related to prostate cancer. In early stages of prostate cancer, the hormone testosterone binds to the ordered part of the protein, where it stimulates cancer growth. Current drugs therefore target and bind to this ordered part, where they block the growth.’
In advanced prostate cancer, however, the cancer cells produce receptor proteins that lack the ordered part. This means the drugs can no longer bind to the protein and the disordered receptor then drives cancer growth undisturbedly while drugs are unable to suppress it.
Current approaches are insufficient
‘Currently used experimental and computational approaches are not suited for studying disordered proteins,’ says Mashaghi. ‘Even recent breakthroughs in computational efforts, such as AI predictions on 3D protein structure were also not able to resolve the conformation of disordered proteins.’
Finding order in the chaos
Mashaghi: ‘The question I’ll address with this grant is as follows,’ says Mashaghi. ‘How to drug a dynamic chain, in order to make its behaviour healthy instead of pathogenic? In order to do that, we first need to understand the dynamics of these chains and secondly, we need to find a way to alter the unfavourable dynamics into the desired dynamics.’
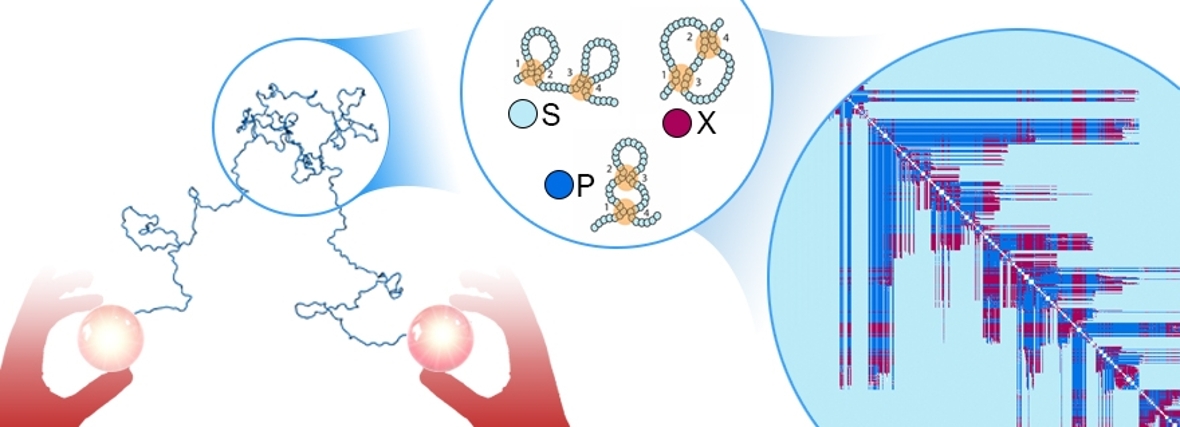
‘For the first problem, we somehow need to find some sort of order in these chaotic and disordered chains. We want to use Circuit Topology (see box) for this, a recently develop set of computational tools that does exactly that: finding order in disordered and changing proteins. The type of order we’re looking for is topological order.’

What is topology?
Topology is an area of mathematics, which studies how spaces are organised, how they are structured in terms of position and how they are connected to each other. Topology has been called rubber-sheet geometry. In a topology of two dimensions there is no difference between a circle and a square. A circle made out of a rubber band can be stretched into a square. However, there is a difference between a circle and a figure eight. A figure eight cannot be stretched into a circle without tearing. In the same way, topologists often joke that a donut is the same as a coffee cup.
See Leiden scientists develop topological barcodes for folded molecules to learn more about Mashaghi’s Circuit Topology method.
Copying Mother Nature
For the remaining problem, the team was inspired by how nature modulates the dynamics of a folding chain using sophisticated biomachines called molecular chaperones. These molecules in our cells regulate the dynamics of a folding protein chain by grabbing it with their appendages or enclosing the entire chain.
‘After finding topological order in these disordered proteins, we want to build artificial molecular chaperones that can control protein topology,’ says Mashaghi. ‘These artificial chaperones can then function as a new type of drugs, that suppress a bad topology and promote a good one. If successful, we can apply our approach to many other undruggable proteins.’
NWO Open Competition – XS grants
The NWO Open Competition - XS grants are yearly awarded by the Dutch Research Council. The XS category emphatically strives to encourage curiosity-driven and bold research involving a relatively quick analysis of a promising idea. This year 26 applications were granted.
Two grants for the Mashaghi lab
Mashaghi’s Medical Systems Biophysics and Bioengineering group at the Leiden Academic Centre for Drug Research received two grants of which the first is described in the article above. The second award is related to single cell analysis of cancer cells that circulate in patient blood, a project which is being conducted by Yasmine Abouleila, a postdoctoral fellow from the Alireza Mashaghi lab.
