
Minute molecular movements might lead to more efficient biofuel cells
Leiden researchers have found minute movements in the laccase enzyme. This discovery could lead to the development of much more efficient biofuel cells. Publication in Biophysical Journal.
The laccase protein enzyme is a very efficient catalyst, which makes it interesting for use in biofuel cells. The laccase enzyme is able to efficiently create water from oxygen without creating hydrogen peroxide, an unstable compound that would damage biofuel cells. Because proteins tend to be unstable, scientists have tried to recreate laccase’s properties in more durable inorganic compounds.
While these new inorganic compounds are sturdier than the original proteins, they are not as efficient. A new discovery by Leiden chemists opens up new possibilities to improve the inorganic replicas.
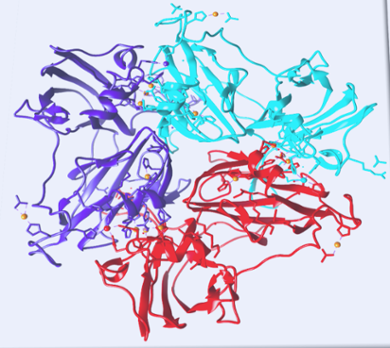
Up until now, scientists considered laccases to be rather rigid and have made inorganic replicas to be similarly firm. Chemist Rubin Dasgupta and others at the Leiden Institute of Chemistry (LIC) have discovered minute motions inside the protein which might contribute to laccase’s efficiency.
Microseconds
These tiny movements happen at the enzyme’s so-called active site, the exact point on the protein where chemical reactions take place. Rubin Dasgupta explains how he discovered the tiny movements: ‘We used nuclear magnetic resonance spectroscopy to look at the active site of the laccase protein. We discovered that laccase moves ever so slightly. These movements only take milliseconds which suggests they could be involved in the reaction.’
According to Dasgupta, these dynamics might be responsible for guiding protons and electrons to the right place during a chemical reaction. Because the inorganic replicas are designed to be rigid, they lack the possibility to guide particles in the same way. This may partially explain their lower efficiency compared to natural laccase.
Bacterial protein
Laccases were first discovered in the nineteenth century, but the specific protein Dasgupta studies was discovered in 2004. While most studied laccases come from fungi, this particular enzyme came from the bacterium Streptomyces coelicolor.
Dasgupta: ‘We chose this particular protein because it has a few advantages over fungal laccases. Normal laccases become inactive in the presence of salt or high alkalinity. This Streptomyces protein stays active in salt or alkaline conditions. If we are able to create an inorganic replica that mimics both the small movements and is resistant to high pH, it will be possible to design a whole new range of efficient biofuel cells.’
Publication
Dasgupta et al. - Chemical exchange at the tri-nuclear copper centre of small laccase from Streptomyces coelicolor. Biophysical Journal (2020)
