
Light and nanoparticles against cancer
Leiden PhD student Xuequan Zhou has designed a new promising molecule that efficiently kills cancer cells, but does not harm healthy tissue. The trick: the drug is only active when irradiated with light. Zhou’s new compound does this extra efficiently by cleverly self-organising into nanoparticles. The research made it to the front cover of the Journal of the American Chemical Society.
Fighting cancer with light
Regular anticancer drugs often make too little distinction between bad and healthy cells: they kill both types. Therefore, the Bonnet group at the Leiden Institute of Chemistry (LIC), focuses on designing new molecules that only become active under the influence of visible light. This allows doctors to treat a certain area of the body, without harming the rest. This so-called photodynamic-therapy is already being used in clinics to fight cancer.
A new medicine
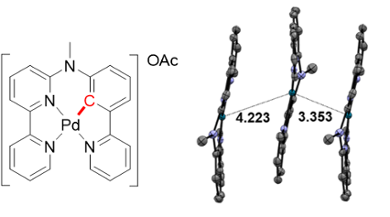
‘The structure of a molecule determines it’s physical, chemical and biological properties’, explains Xuequan Zhou. ‘Therefore, changing this structure can have a huge impact on its performance. Our new work is a great example of this.’ Zhou created a new and efficient anticancer compound by making just a small change in an existing molecule: he replaced one nitrogen atom for a carbon. This resulted in a molecule containing palladium as a metal centre, directly bound to an organic fragment via a carbon-palladium bond (figure 1). Because of this bond, the molecule reacts to blue light and can perform excellent cell-killing when irradiated with this blue light (See frame).
Light induced therapy
Xuequan’s palladium species works through so-called oxygen activation. When irradiated with light, the palladium complex gets into an excited state (meaning it gets extra energy). The excited palladium complex then transfers this light energy to a dioxygen-molecule (O2) which is present in the irradiated cell or tissue. This generates reactive oxygen species that then kill the cell.
Self-assembling nanoparticles
‘Besides its photochemical behaviour, this molecule also shows quite extraordinary aggregation properties’, tells Zhou. ‘Because of its low charge and rather hydrophobic organic ligand, it has a tendency to self-assemble via a process called “metallophilic interaction”: the palladium centres love each other and try to be close to one another.’ When dissolved in the body, this would cause Zhou’s compound to self-assemble into nanoparticles.
Cancer cells can absorb these blue-light activated nanoparticles very efficiently. They are therefore used as cancer-targeting nanoparticles: ‘Normally, these nanoparticles are specifically being attached to anticancer compounds in order to help them target a tumour’, explains supervisor Sylvestre Bonnet. ‘For the new compound of Zhou however, this step is no longer necessary, because the drug itself creates its own nanoparticles.’
Promising results
With the first biological experiments in Leiden, Zhou already demonstrated in vitro that the nanoparticles indeed are very efficient at killing cancer cells under blue light irradiation. Next, a collaboration with professor Wen Sun from Dalian University of Technology in China showed that the nanoparticles can inhibit cancer growth in mouse tumour models. Zhou: ‘Altogether, these results suggest a promising future for self-assembling molecules as anti-cancer drugs, which can better target tumours and hence eradicate them more efficiently.’
