Research project
Light-activatable metallodrugs and metal-functionalized liposomes
Metal-containing molecules combine geometrical features and a reactivity that are inherently different from that of organic molecules. My research focuses on light-activatable metal-based anticancer drugs and metal-functionalized liposomes. Light is a very selective way to activate photosensitive drugs in vivo. In cancer research killing diseased cells selectively without killing the healthy cells nearby is a difficult challenge. Using light to control when and where a molecule will interact with biomolecules and kill the cell, holds promise in reducing side effects of chemotherapy. Meanwhile, functionalizing lipid nanovesicles with such metal complexes allows for targeting the metal compounds to tumors. Metal-functionalized liposomes are also developed that can sense small analytes, or store solar energy into chemical bonds.
- Contact
- Sylvestre Bonnet
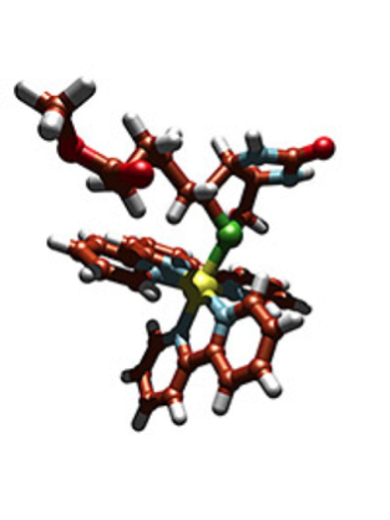
Organic ligands and a metal ion bound together form a “metal complex”. Once introduced in a biological environment (serum, a cell, or a biomimetic environment) these metal complexes may in turn bind to biological ligands such as lipids, aminoacid residues of proteins, nucleic acids (DNA), or small molecules (vitamins, ions, neurotransmitters, etc.). The interactions between the metal complex and these biomolecules depend on the details of the organic ligands and on the nature (charge, oxidation state) of the metal ion. Overall, we study coordination chemistry in biological and biomimetic environments, with applications in chemical biology, medicine, and energy.
Light activatable anticancer metallodrugs
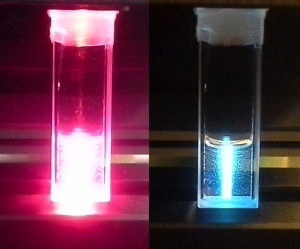
Ruthenium polypyridyl complexes are a class of molecules known for their rich photochemistry. Meanwhile, it was discovered several years ago that they can have anticancer properties if the ligands around the metal center have the appropriate formula. We combine both properties to obtain molecules that have a low toxicity in the dark, but recover a high toxicity against cancer cells upon visible light irradiation. Activation takes place via the selective rupture of a ruthenium-ligand chemical bond: in the dark the stable Ru-L bond prevents the complex of interacting with biomolecules, whereas after light irradiation the protecting ligand L is cleaved away, which recovers the metal’s ability to bind to biomolecules such as DNA, lipids membranes, or proteins, and thus triggers phototoxicity. Meanwhile, these metal complexes are also chemical bound to cancer-targeting molecules or drug delivery systems (for example, liposomes), with an aim of increasing the concentration of the prodrug in the tumor before light irradiation is performed.
Upconverting drug delivery systems
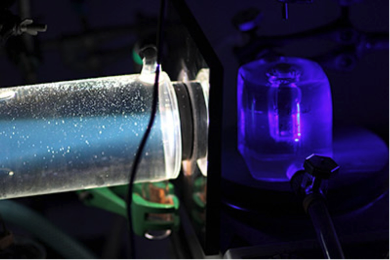
Not any kind of light penetrates human tissue deep enough to allow treating large tumors in cancer patients. Red and near-infrared light penetrates tissues better than blue light, which is absorbed by blood and melanin. As ruthenium polypyridyl complexes often need blue or green light to be photochemically activated we develop upconverting drug delivery system that are able to convert in situ low-energy photons into high-energy photons. For example, upconverting liposomes have been obtained that can convert red light from a clinical-grade laser (630 nm) into blue light (473 nm). The upconverted blue light is generated locally and does not need to travel over long distance before it is absorbed by the photosensitive prodrug. By mixing such upconverting liposomes with liposomes functionalized with anticancer ruthenium-based prodrugs, activation of the metallodrug takes place efficiently although the metallodrug itself does not absorb red light!
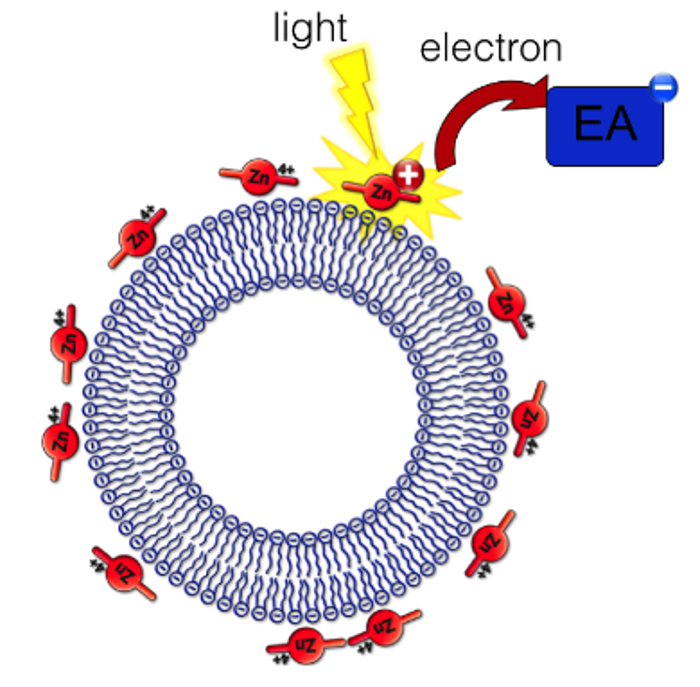
Metal-functionalized liposomes for sensing or solar fuel production
By chemical functionalization of metal-binding organic ligands, or by simply using electrostatic interaction between positively charged metal complexes and negatively charged lipids it is possible to functionalize liposomes with metal complexes, or sense metal ions binding to the surface of the bilayer. We also study the motion of molecules at the surface of lipid bilayers using light. Another project uses metal-functionalized lipid bilayers to increase the efficiency of charge separation in artificial photosynthesis, and possibly obtain efficient photocatalysis at lipid bilayers. Charged liposomes were for example shown to have a dramatic influence on the efficiency of electron transfer at the membrane surface.
Key publications
- “The self-assembly of a cyclometalated palladium photosensitizer into proteins-stabilized nanorods triggered drug uptake in vitro and in vivo”, X.-Q. Zhou, M. Xiao, V. Ramu, J. Hilgendorf, X. Li, P. Papadopoulou, M. A. Siegler, A. Kros, W. Sun*, S. Bonnet*, J. Am. Chem. Soc. 2020, 142, 10383-10399
- “Mimicking Photosystem I with a Transmembrane Light Harvester and Energy Transfer-Induced Photoreduction in Phospholipid Bilayers”, A. Pannwitz,* H. Saaring, N. Beztsinna, X. Li, M. A. Siegler, S. Bonnet*, Chem. Eur. J. 2020, https://dx.doi.org/10.1002/chem.202003391.
- “Photo-uncaging of a microtubule-targeted rigidin analogue in hypoxic cancer cells and in a xenograft mouse model”, V. H. S. van Rixel, V. Ramu, A. B. Auyeung, N. Beztsinna, D. Y. Leger, S. T. Hilt, S. E. Le Dévédec, T. Yildiz, T. Betancourt, M. B. Gildner, T. W. Hudnall, V. Sol, B. Liagre, A. Kornienko,* S. Bonnet*, J. Am. Chem. Soc. 2019, 141, 18444-18454
- “Why developing PhotoActivated Chemotherapy?”, S. Bonnet*, Dalton Trans. 2018, 47, 10330-10343, Special issue “New Talent 2018: Europe”
- “Solving the oxygen sensitivity of sensitized upconversion for life science applications”, S. H. C. Askes, S. Bonnet*, Nat. Rev. Chem. 2018, 2, 437-452
- “Efficient red light activated NAMPT inhibition in hypoxic cancer cells using water-soluble ruthenium complexes”, L. N. Lameijer, D. Ernst, S. L. Hopkins, M. S. Meijer, S. H. C. Askes, S. E. Le Dévédec, S. Bonnet*, Angew. Chem. Int. Ed. 2017, 56,11549–11553
