Research project
Using zebrafish to target the Achilles’ heel of cancer
Exploiting metabolic vulnerabilities to identify anticancer compounds in zebrafish synthetic lethality screens.
- Duration
- 2012 - 2017
- Funding
-
 KWF (Dutch Cancer Society)
KWF (Dutch Cancer Society)

The pursuit for targeted cancer therapy is aimed at identifying drug targets that are essential in cancer cells but not in normal cells. Such targets are defined as cancer-specific vulnerabilities or as synthetic lethal interactions with cancer-specific genetic lesions. It has been appreciated that cancer cells undergo fundamental changes in their metabolism (metabolic transformation) in order to meet their increased energetic and biosynthetic demands ( Figure 1). However, the molecular mechanisms that underlie this transformation are not well understood.
The altered metabolic characteristics of cancer cells distinguish them from normal differentiated cells and provide vulnerability points exclusive to the cancer cells that can be targeted for efficient treatment. Indeed, it has been proposed that cancer metabolism constitutes an Achilles’ heel of cancer.
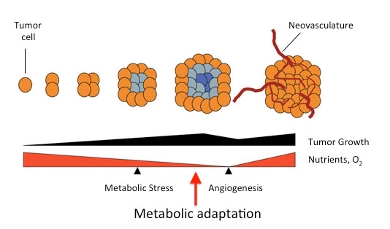
As a tumor grows and before recruitment of blood vessels from the host (angiogenesis) begins, cancer cells at the center of the tumor are deprived of nutrients and oxygen. Tumor cells reprogram their metabolism (metabolic adaptation/transformation) in order to grow and proliferate in a nutrient and oxygen-poor environment. Scheme adapted from (Jones and Thompson, Genes and Dev, 2009).
In this project, we use zebrafish deficient in the tumor suppressor Lkb1 which exhibit deregulated metabolism, to identify pathways whose combined inhibition with lkb1 deficiency lead to (synthetic) lethality.
Synthetic-lethality screens have primarily been conducted in cell-based assays. However, while in vitro assays have provided valuable information, they are often limited in that they cannot fully recapitulate the complexity of the tumor-microenvironment interactions that occur in vivo. The zebrafish is emerging as a valid model to discover pathways relevant in human cancer and provides an ideal setting to conduct chemical-genetic screens for synthetic-lethal interactions in the intact organism.
The tumor suppressor LKB1 is mutated in a familial cancer-predisposition syndrome and in over 30% of lung adenocarcinomas, one of the most common human tumors with a very poor prognosis. LKB1 is a serine-threonine kinase that phosphorylates the AMP-activated protein kinase (AMPK), a key energy checkpoint at the cellular and organismal levels. AMPK activation leads to growth suppression through several pathways including inhibition of the mTOR pathway. We previously showed that Lkb1 is critical to maintain energy homeostasis in zebrafish (Figure 2). Lkb1 zebrafish mutants are embryonic-viable (in contrast to mice), exhibit deregulated metabolism and fast metabolic rate, a characteristic of tumor cells.
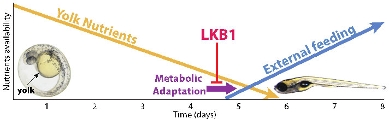
During zebrafish development, there is a transition from a high-energy state (left image: 1day-embryo, arrow indicates abundant yolk) to a condition of restricted resources (right image: 6 day-larva with depleted yolk supply). The metabolic stress accompanying the depletion of yolk-derived nutrients induces an adaptation program. The tumor suppressor LKB1 orchestrates this metabolic adaptation.
In this project we will perform chemical-genetic screens in the intact organism in vivo, using our lkb1 zebrafish model of deregulated metabolism. We will look for compounds/pathways that can kill selectively the lkb1 mutants but not wild-types. We will use libraries of compounds with known biological activity, consisting mainly of protein kinase and phosphatase inhibitors. In our preliminary work, we have identified a few compounds that lead to premature lethality of selectively the lkb1 mutants while their wt siblings are largely unaffected (Figure 3). We are currently validating these leads and investigating their mechanism of action.
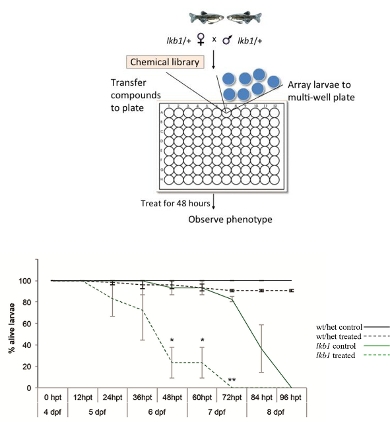
Wt, lkb/+, and lkb1 homozygote larvae at 4 days post fertilization (dpf) are arrayed on a multi-well plate containing different compounds. The larvae are treated with the compounds for two days and subsequently scored for abnormalities and/or lethality.
Survival curve of wt/het and lkb1 mutant larvae treated with DMSO (control) or a compound that activates metabolism. The compound has a minimal effect on the survival of wt larvae but a profound effect on the survival of lkb1 larvae. After two days of treatment, 80% of the lkb1 mutants have died. The data depicted here represent three independent experiments with at least 100 larvae used/experiment. hpt: hours post-treatment.
