
New insight brings sustainable hydrogen one step closer
Leiden chemists Marc Koper and Ian McCrum have discovered that the degree to which a metal binds to the oxygen atom of water is decisive for how well the chemical conversion of water to molecular hydrogen takes place. This insight helps to develop better catalysts for the production of sustainable hydrogen, an important raw material for the chemical industry and the fuel needed for environmentally friendly hydrogen cars. Publication in Nature Energy.
Heated debate
For years there has been a heated debate in the literature: how to speed up the electrochemical production (see box) of hydrogen on platinum electrodes in an alkaline environment? Chemist Ian McCrum watched from the sidelines and concluded that part of the debate was caused by the fact that the debaters were looking at slightly different electrodes, making the results incomparable. Time to change that, McCrum thought, who was a LEaDing Fellow postdoc in the group of Professor Marc Koper at the time.
Platinum crystal
McCrum, who now works in America, used a special platinum crystal. To understand what is so special about this crystal, we need to zoom in on the surface of the platinum. This is not flat and smooth, but irregular with tiny steps and kinks. And it is precisely at these irregularities that chemical reactions take place. McCrum designed the special crystal in such a way that the surface has the same number of these irregularities throughout the crystal. He then decorated the edges with different metals, such as ruthenium and molybdenum. In this way, he ensured that all the electrodes had exactly the same atomic structure, but each time with a different metal in the edges. This enabled him to vary the interaction of the electrode with the oxygen atom of water in a systematic and well-defined way.
Surprising outcome
Measurements then began, with a surprising outcome. Marc Koper: ‘Our breakthrough is that there appears to be a clear link between the activity of the electrode for making hydrogen and the degree to which the metal in the edge binds to the oxygen atom of water.’ The latter is also known as oxophilicity, with oxophilic literally meaning oxygen-loving. ‘We have even found an optimum for this oxophilicity,’ says Koper. ‘We have now definitively established that the oxophilicity of the surface plays a very important role in electrolysis.’ The scientists also developed a model to explain the existence of this optimum.
Towards sustainable hydrogen
The findings are a big step forward in the scientific debate. Koper: ‘This new knowledge is important in our field. Because we have found an optimum in oxophilicity, we can look more specifically for better catalysts for the sustainable production of hydrogen.’
Publication
Ian McCrum & Marc Koper, The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum, Nature Energy (19 October, 2020)
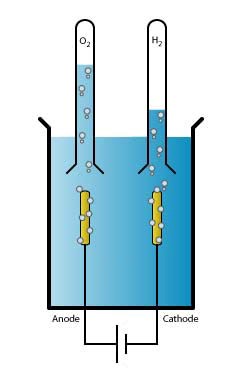
Elektrochemistry
Electrochemistry is a field that focuses on the relationship between electricity and chemistry. For example, the conversion of energy into batteries belongs to the field of electrochemistry. Another well-known example is the electrolysis of water, in which oxygen and hydrogen gas are formed – exactly the reaction that Marc Koper's group focuses on. There are two electrodes which run a current through the water: the positively charged anode and the negatively charged cathode. Eventually, molecular oxygen is formed at the anode and molecular hydrogen at the cathode.
