
Conversion of renewable raw materials on platinum shows unexpected behaviour
The electrochemical reduction of a group of organic compounds on platinum is strongly dependent on the arrangement of the atoms in the platinum surface. Christoph Bondue, postdoc in Marc Koper's group, published this in Nature Catalysis on 4 March. The reduction of such compounds is an important process in making chemical raw materials more sustainable.
Sustainable raw materials
By far the most raw materials in the chemical industry are based on fossil sources, making them inherently unsustainable. A more sustainable alternative would be to switch to biomass-related raw materials, for example in the synthesis of plastics. Compared to fossil hydrocarbons, biomass contains a lot more C=O bonds: a carbon and oxygen atom with a double bond. Such a compound with a C=O bond is also called a ketone. Before this biomass can be used in existing chemical processes, the C=O bonds must be reduced.
Sustainable reduction
'If you can carry out this reduction using sustainably generated electricity, in other words with electrochemistry, that would be great', says Marc Koper, Professor of Catalysis and Surface Chemistry. 'That is what we have focused on in this study.' The research is part of an NWO project in the context of 'New Chemical Innovations', co-funded by Shell and Akzo Nobel.
Nail polish remover
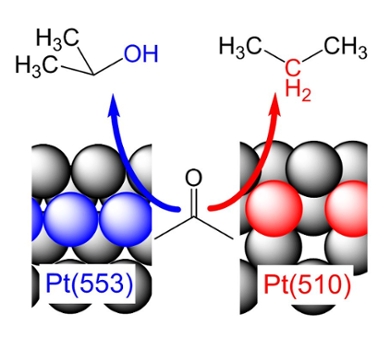
For this research, Bondue and Koper collaborated with Federico Calle-Vallejo of the University of Barcelona. Calle-Vallejo supported the experimental work with computer calculations. The researchers looked at the simplest ketone compound, the solvent acetone - better known to most people as nail polish remover. They looked at the possibility of reducing the C=O bond using electrochemistry, with platinum as an electrocatalyst. The atomic structure of the platinum surface affected not only the effectiveness of the reaction, but also the outcome.
Surface determines
For instance, nothing at all happens on a platinum surface in which the atoms are most closely packed, in the honeycomb structure. On a surface in which the platinum atoms are arranged like a chessboard, a product is formed that no longer wants to leave the surface: such a platinum surface inactivates itself. The actual reduction of the C=O bond only takes place if there are so-called defects in the surface structure. These are interruptions in the regular honeycomb or chessboard structure.
Defects
The catalysis happens in such a way that the product of the reduction depends on how exactly the platinum atoms are arranged in the defect. To describe this in more detail, chemist use the coordination number. This indicates to how many other atoms a platinum atom is connected. Koper and his colleagues found that a defect with a high coordination number produces 2-propanol, an alcohol. On a defect with a lower coordination number, the researchers measured propane – a molecule in which the oxygen atom originally contained in acetone has reacted away completely.
Unique outcome
'It has been reported regularly that the reactivity is very sensitive to the structure of the platinum surface,' says Koper. 'See for example the recent research by Ludo Juurlink, who used a curved platinum surface. But the observation that the selectivity - or the product you make - is so sensitive to the local structure is quite unique.'
'With this insight, we can more efficiently develop catalysts for the conversion of biomass-related molecules into desired end products', says Koper. 'Our recent experiments show that more complex ketones than acetone exhibit the same behaviour, such as the reduction of acetophenone, an aromatic fragrance.' This is an indication that the research of Koper and his co-workers can be extrapolated to the reduction of biomass.
