
New research shows the limitations of coordination in chemistry
A common assumption in chemistry is that the coordination number of a catalyst's surface determines the reactivity of the reaction it catalyses. Strikingly, Leiden chemists have now proven that this is not true for nature’s most simple chemical reaction: the dissociation of hydrogen. The researchers managed to measure the so-called absolute reactivities of this reaction, a first in science. Publication in Angewandte Chemie.
Secret weapon
Catalysts are widely used in our daily lives, such as the platinum catalyst in your car exhaust. However, it is often not understood how they exactly work. Professor Ludo Juurlink and his group try to change this and have a secret weapon to do so: a curved platinum surface. Last year, this piece of equipment earned them two publications in leading journals: in Science they finally proved which theoretical model for the reaction of hydrogen on platinum is correct and in PNAS they showed how oxygen reacts on platinum. Now, the chemists used this special platinum to shake up an important established idea in chemistry: coordination.
Zooming in on a catalyst
To explain this, we first have to take a look at the surface of a catalyst, which is where chemical reactions take place. This surface consists of atoms – platinum atoms in this study – which are aligned in a specific way. Each atom has a different coordination number, indicating how many other atoms surround this particular atom. If we take the reaction of hydrogen on platinum as an example, the general idea was as follows: the coordination number of the atoms on the surface of platinum determines the reactivity toward molecular hydrogen.
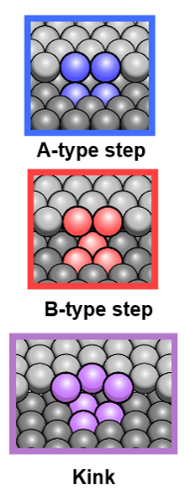
The Leiden team used two curved platinum crystals to test this hypothesis. ‘Because the platinum surface is curved, the atomic structure changes very gradually along the surface,’ explains group leader Juurlink. ‘You can compare this structure to a staircase, whose steps towards the edges become narrower and narrower. In the middle, it looks more like a ballroom.’ But why is that important? A catalyst’s surface is not flat and smooth, but irregular, with steps and kinks. And exactly at these irregularities, chemical reactions take place. With the curved platinum, the researchers mimic this effect, whilst simultaneously knowing exactly how many steps or kinks each part of the crystal has. This enabled the chemists to measure the reactivity of hydrogen compared to the density of steps or kinks on platinum.
Steps and kinks
‘We identified three types of irregularities,’ says PhD candidate Sabine Auras, first author of the study. ‘There are the A-type, the B-type, and the kinked type.’ For each type, she measured the reactivities. Where the surface has more steps or more kinks, the reactivity increases. So far, nothing new under the sun. ‘But we also found that this increase is different for each type of irregularity. And this difference does not correspond to what one would expect in terms of coordination.’ Instead, the team defined cross-sections for the interaction of hydrogen and steps or kinks, which are an important landmark for other researchers in the field.
‘You cannot always steer science’
Long-term vision
The foundations for this publication in Angewandte Chemie were laid five years ago when former PhD candidate Dima Bashlakov discovered something special when studying a curved platinum crystal. ‘I think our research endorses the idea that science funding should never be focused on short-term vision or short-term deliverables,’ says Juurlink. ‘Sadly enough, these are both aspects that seem so common in research applications these days.’ Auras adds: ‘I understand that we have a responsibility to society. But with a certain degree of freedom, which long-term funding can provide, we can get really unique results. You cannot always steer science.’
Publication
Sabine Auras et al. Scaling Pt-catalyzed hydrogen dissociation on corrugated surfaces, Angewandte Chemie (2020).
Text: Bryce Benda
Header image: Hydrogen in its plasma state. © Alchemist-hp.
