We finally understand how oxygen reacts on platinum
Platinum is a widely used catalyst, but its precise mechanism largely remains a mystery to scientists. Ludo Juurlink has now demonstrated for the first time how oxygen reacts on the platinum surface. Together with PhD students Kun Cao and Richard van Lent and international colleagues he publishes his findings in leading journal PNAS.
Juurlink strikes again
Earlier this year, Juurlink managed to solve a forty-year-old problem in chemistry together with Richard van Lent and the DIFFER institute. Using a unique curved platinum surface – for which Juurlinks lab is internationally renowned – he proved how hydrogen reacts on platinum. In his current research, he again uses the curved platinum, this time investigating the reaction with oxygen.
How does oxygen react?
This has led to an interesting discovery. Juurlink and colleagues observed that oxygen reacts on platinum in a different way than the much lighter hydrogen. The curved platinum was again crucial for this discovery. 'Because the platinum surface is curved, the atomic structure changes very gradually along the surface,' explains Juurlink. This structure can be compared to a staircase with steps that become narrower and narrower towards the edges. In the middle, the surface looks more like a ballroom'.
The reactivity of hydrogen turned out to depend on how close the steps of the catalyst are to each other. This is also the case with oxygen, but for a fundamentally different reason. 'The steps have a different effect on oxygen than on hydrogen.'
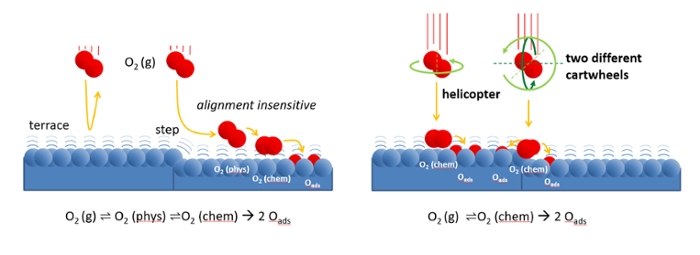
Figure left: Oxygen molecules approach the catalyst surface with low velocity. The steps on the surface scatter the molecules onto a weakly bound ‘physisorbed’ state. From there, the molecules may easily find their way to a site where they bind chemically and finally dissociate. On the atomically flat terraces, scattering back into the gas phase is much more likely.
Because the physisorbed state is weakly bound, it strongly resembles the molecule in the gas phase. In both, the molecule may rotate. The scattering process therefore does not depend on the molecule’s initial alignment.
Figure right: At high incident velocity, O2 may directly be adsorbed into a chemically bound state. At terraces, molecules that helicopter have a higher chance to bind than those that ‘carthweel’. Only at steps, cartwheeling molecules are sensitive to the alignment. Molecules that rotate along the steps edge (dark green rotation) more easily stick to the edge than those rotating against the edge (light green rotation).
Unique lab in Japan
According to Juurlink, this mainly has to do with the larger mass of oxygen. 'Because oxygen is heavier than hydrogen, the interaction with the platinum surface starts from a greater distance,' he says. 'The oxygen molecule already feels the interaction with platinum, but cannot yet see the details. As a result, the reaction takes place in a different way than with hydrogen.'
For the experiment it was necessary to be able to control the direction of rotation of the oxygen molecules. This required cooperation with a Japanese colleague – Mitsunori Kurahashi – who built a unique device for this purpose. 'Last year, I had the opportunity to carry out measurements in his lab for two weeks on a grant from the institute where Kurahashi works,' says Juurlink.
Why is this discovery important?
A beautiful fundamental discovery, concludes Juurlink, which may also have an impact on existing applications. The reaction of oxygen to platinum is essential in the sustainable energy sector and in improving air quality. 'For example, the reaction takes place in hydrogen fuel cells and in car exhaust systems,' says the chemist. 'The fact that we can now measure how the reaction proceeds at such a detailed level, poses challenges to theoretical models that describe this chemical reaction and make predictions about it.’
Read the paper in PNAS: Steps on Pt stereodynamically filter sticking of O2
