
Chemists solve persistent problem after four decades
After almost four decades, Leiden and Eindhoven chemists have resolved the discussion about the correct model for the simplest chemical reaction in heterogeneous catalysis, which is essential for fuel cells. Using a unique curved platinum surface, Ludo Juurlink and PhD candidate Richard van Lent from Leiden and Michael Gleeson from DIFFER showed which model correctly describes the reaction of hydrogen. They published their findings in Science on 11 January.
Endless discussion
For almost four decades there has been a heated debate in the chemical literature: which of the two existing models for the reaction of hydrogen to a platinum catalyst is correct (see box)? Traditional methods were not adequate to prove this. Leiden chemist Ludo Juurlink and Michael Gleeson from the Dutch Institute for Fundamental Energy Research (DIFFER) decided to develop a new method to provide conclusive evidence, which proved to be successful.

A staircase that gets narrower and narrower
The two existing models for heterogeneous catalysis give different predictions about how the reaction of hydrogen depends on the structure of the platinum surface. By means of measurements the researchers were able to determine the reactivity of hydrogen and thus prove which model is correct. The curved platinum crystal, which was made for the first time by a Dutch company in Zaandam, was crucial for this. ‘Because the platinum surface is curved, the atomic structure changes very gradually along the surface,' explains Juurlink. ‘You can compare this structure to a staircase, whose steps towards the edges become narrower and narrower. In the middle it looks more like a ballroom.’ It turned out that the reactivity of hydrogen was linearly dependent on how close the steps are to each other. The further apart the steps were, the less reactive hydrogen was. ‘And so the model that predicted a non-linear behaviour is incorrect,' he says.
Unique possibility
The research was conducted in ultra-high vacuum and provides important insights. 'We now know better how to calculate the speed of chemical reactions - one of the models does not contribute significantly,' says Juurlink. 'In addition, we now know that these curved crystal surfaces offer a unique, new opportunity to learn how chemical reactions actually occur on surfaces. We are certainly going to do more research on that'.
Why does a catalyst work?
Almost all major chemical industrial processes use heterogeneous catalysis. Catalysts are sometimes expensive and rare, as in the case of platinum, a common catalyst found in fuel cells and car exhaust systems. 'The unusual thing is that we usually don't even really know how and why such catalysts speed up chemical reactions,' says Juurlink. Better insight into the how and why of this will contribute to making the chemical industry more sustainable. Once we have a better understanding of what is happening at atomic level, we can develop new catalysts,' says Juurlink. 'Catalysts that cause less energy loss and are less dependent on expensive and rare materials'.
Publication
Site-specific reactivity of molecules with surface defects—the case of H2 dissociation on Pt
Richard van Lent, Sabine V. Auras, Kun Cao, Anton J. Walsh, Michael A. Gleeson, Ludo B. F. Juurlink
Science, 11 Jan 2019: Vol. 363, Issue 6423, pp. 155-157 DOI: 10.1126/science.aau6716
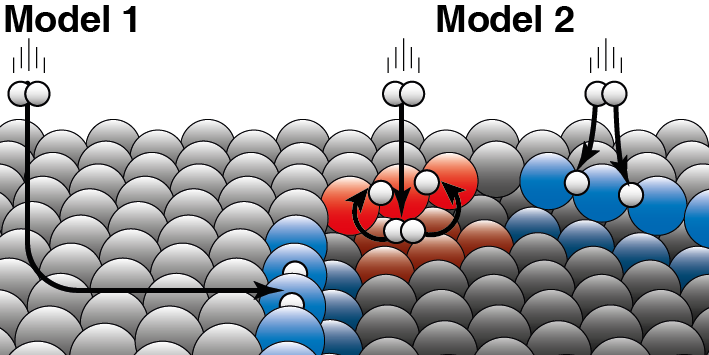
A catalyst accelerates a chemical reaction without being consumed itself. In heterogeneous catalysis, the catalyst is usually a solid and the reactants a gas or liquid. Juurlink explains the difference between the two models: 'The two models are based on different assumptions about how kinetic energy from the hydrogen molecule "leaks away" during the collision with the platinum surface.' To clarify this, he gives an example: 'If a dog is in an ice hole, he may have ended up there in two ways. Either he slipped over the ice from the side and fell into the ice hole, or he jumped directly into the ice hole from the side.' Their research now shows that hydrogen molecules mainly react directly out of the gas phase at the step edge of platinum (Model 2). Model 1, which assumes that most molecules end up at the edges by 'skating' over the flat platinum surface and only then react, is therefore not correct.
The researchers saw differences between the two different types of edges (red and blue) that occur naturally on platinum catalysts. Hydrogen can land on the top or bottom of such an edge. For both types of step edges, the researchers are able to decide which part reacts directly on the upper side (as illustrated in blue) or first lands on the lower side (as in red).
