
Two cum laude distinctions for storing renewable energy
Both Leon Jacobse and Thom Hersbach from Marc Koper's research group obtained their PhDs cum laude. They both investigated changes on the surface of a platinum electrode. Jacobse studied this at a positive voltage, Hersbach at a negative voltage. Platinum has the potential to convert renewable energy using water into hydrogen, which can then be used as fuel.
Storing renewable energy as hydrogen
Sustainable energy, such as solar and wind energy, is not always available in the right place and at the right time. In order to have access to renewable energy anywhere and anytime, it is important to find ways to store it. Electrolysis can help: with electrolysis, water and sustainable electricity can be converted into hydrogen and oxygen. The energy stored in hydrogen can then be used, for example by means of a fuel cell in a hydrogen car. Platinum plays an important role in both reactions.
Platinum not yet understood
Despite the fact that platinum is frequently used – for instance in the exhaust of current cars – scientists still do not understand exactly how it behaves. Both Jacobse and Hersbach have done fundamental research during their PhD, with the main aim to better understand the electrochemical (see box) behaviour of platinum. They both studied structural changes on platinum electrodes and the effects these changes have on the reactivity of these electrodes.

Rusty research
'I have investigated how a platinum surface is affected by oxidation at a positive voltage', says Jacobse. Oxidation is the same process as the rusting of your bike. 'From the literature it was already known that this oxidation changes the reactivity of platinum, but how the reactivity and the structure of the surface relate to each other was still unknown.' Jacobse used Electrochemical Scanning Tunneling Microscopy, a technique in which a needle of only a few atoms thick scans the platinum surface. 'Unique to our experiment is that we did this in an electrochemical cell', he says. 'Only in this way it was possible to follow the changes in the structure step by step and to relate them directly to the reactivity we measured in the same experiment.'

Absorbing electrons
'I focused on so-called cathodic corrosion', says Hersbach. 'This is the corrosion of metals such as platinum that does not occur at a positive voltage, as with Leon, but at a negative voltage. A phenomenon that is not yet understood, but only a handful of people worldwide is working on it. A field where a lot of new knowledge can be gained.' Hersbach discovered that cathodic corrosion occurs at a much lower voltage than was thought. And that means that cathodic corrosion probably influences far more reactions than was suspected. 'I have also shown that cathodic corrosion can improve platinum's catalytic activity for the conversion of oxygen to water, an important reaction in fuel cells.'
Meanwhile, both chemists have found a postdoc position. Jacobse has been working at German Electron Synchrotron (DESY) in Hamburg since December, Hersbach starts in February at the University of Texas in Austin, America.
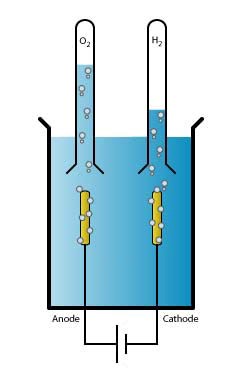
Electrochemistry is the field that focuses on the relationship between electricity and chemistry. For example, the conversion of energy into batteries falls under electrochemistry. The simplest example is the electrolysis of water, where oxygen and hydrogen are formed – exactly the reaction that Marc Koper's group focuses on. There are two electrodes which run a current through the water: the positively charged anode and the negatively charged cathode. In an equilibrium reaction, the water splits into a hydrogen ion (H+, or proton) and a hydroxide ion (OH-). At the cathode, hydrogen is formed by the reaction of four protons with four electrons. At the anode, water and oxygen are formed from hydroxide ions.
|
Water splitting: |
H2O |
↔ |
H+ + OH− |
|
Cathode: |
4 H+ + 4 e− |
→ |
2 H2 |
|
Anode: |
4 OH− |
→ |
2 H2O + O2 + 4 e− |
