Mind the gap(s)! A surface science approach to catalysis?
Surface Reaction Barriometry: Methane Dissociation on Flat and Stepped Transition-Metal Surfaces.
The dissociation of methane on a catalyst surface is a crucial step in the steam reforming reaction used for the commercial production of hydrogen. Researchers in the Theoretical Chemistry group at Leiden University have now made a great advance in providing an accurate prediction of this process which is of both fundamental interest and industrial importance.
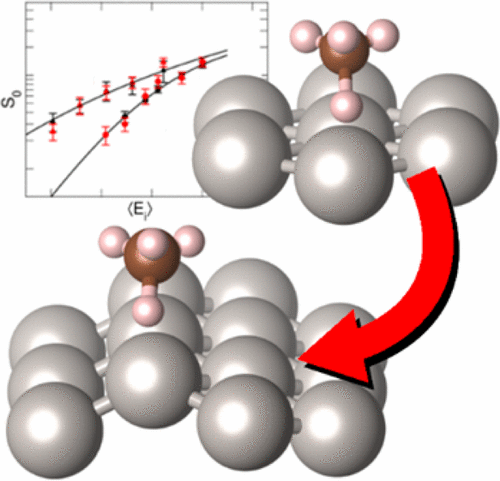
In a recent collaboration with the group of Art Utz (Tufts University, Medford, USA), scientists from the Leiden group have been able to describe the dissociative chemisorption of CHD3 on Ni(111) within chemical accuracy (1kcal/mol or 4.2kJ/mol). This was achieved using a semi-empirical specific reaction parameter (SRP) functional within density functional theory.
By combining the results of molecular beam experiments from the group of Rainer Beck (EPFL, Lausanne, Switzerland) and ab-initio molecular dynamics calculations, they have shown that the same SRP functional also quantitatively (within chemical accuracy) reproduces the initial dissociation probabilities for CHD3 on both Pt(111) and Pt(211).
The work, published in the Journal of Physical Chemistry Letters, is the first time that the transferability of an SRP functional has been demonstrated between different transition metal surfaces, and also from a flat(111) to a stepped (211) surface of one and the same metal, for one and the same molecule interacting with these surfaces.
The applicability of the SRP functional to line defects present on the Pt(211) surface suggests that the functional will also describe the dissociation of CHD3 on other point defects or kinks, which would make it possible to model the dissociation of methane on the defected surfaces of heterogeneous catalysts.
