
Wrestling with SUMO proteins
The work done by SUMO proteins in the cells of our body is of vital importance. Molecular cell biologist Alfred Vertegaal has been studying these proteins for nine years, first with a Veni subsidy and then with a prestigious Vidi subsidy.

A thousand different types
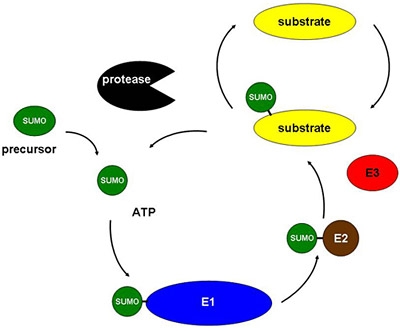
A SUMO (Small Ubiquitin-like MOdifier) is a small protein that can bind to another protein, and can also be released from it. There are some thousand different types of proteins, also known as substrates, to which SUMOs can bind; each of them has its own specific function. SUMOs modify the protein to which they bind, so that it functions in the essential processes of a cell, such as in the transportation or transcription of DNA or the response to stress.
Surprising
SUMOs were discovered not so long ago, in 1996. It may be a coincidence, but this is also the year in which Alfred Vertegaal graduated in Leiden as a biomedical scientist. In 2001 in Dundee, Scotland, he started on a research project on SUMOs funded by the Dutch Cancer Society. He returned to Leiden in 2003 and, together with his research group in the LUMC, won first a Veni and then a Vidi subsidy.
‘It's fantastic’, says Vertegaal, ‘although in the Vidi project we want to do other things than what we have done with the Veni funds.' The Veni project was the impetus for the new project, as Vertegaal by chance came across something rather surprising.
Ubiquitin

Vertegaal: ‘In the Veni project, we investigated what kind of substrates are the focus of SUMO-2 proteins, one of the four SUMOs. When we had a good look at the substrates, as well as an attached SUMO protein, we also discovered a number of ubiquitin proteins. Ubiquitin is frequently involved in the break-down of proteins, which gave us the idea that SUMO-2 might work together with ubiquitin in the break-down of proteins. We have since managed to confirm this new role for SUMO-2. We now want to use the Vidi subsidy to really get to understand the process.'
First, the relevant substrates have to be identified: what kinds of substrates are they exactly, and what is their function? It is already clear that particular substrates are involved in cell division. Then, Vertegaal will look at what exactly the SUMO protein does with the substrate. How does he plan to do this? 'First we will examine the substrate for sites where the SUMO protein can bind,' Vertegaal explains. 'Then we will alter the substrate such that the SUMO protein can no longer bind to it, and we can then compare the two substrates. Hopefully, we will find out why a particular substrate is selected for break-down.'
Biological relevance
As well as a fundamental aspect, explaining this alternative route to break-down, this research also has biological relevance. All the activity takes place in the nucleus of the cell and the research comprises important functions such as cell division and DNA replication. Also, in the future Vertegaal's results may be of benefit to the medical sector. 'You see problems with protein break-down in a number of types of cancer and in conditions such as Alzheimer's and Parkinson's,' Vertegaal explains. 'It is essential to be able to identify those substrates that are no longer properly broken down in cases of these diseases.'
Collaboration
Vertegaal's role extends further than his room in the LUMC. 'Collaboration with other research groups is essential,' he says. 'We only do one part within the whole picture of modifications to proteins in DNA management. If you want to understand all the different types of modifications, then you have to combine forces with other research groups.'
Vertegaal has enough to keep him busy for the time being. ‘It used to be the case that we knew about only a few substrates and modifications. Now we are finding so many different and interesting substrates that we are inundated with work. But I regard it as a challenge to try to understand a cell at this level.'
And finally, an obvious question: is there any connection with sumo wrestlers? 'No,' laughs Vertegaal. 'A SUMO protein is extremely small and in no way comparable to a large, fat wrestler. The most you could say is that we are 'wrestling' with proteins.'
(18 August 2009)
