Research project
Classical conditioning to improve immunotherapy in cancer
Can the conditioning paradigm be used for checkpoint inhibition cancer treatment?
- Duration
- 2022
- Contact
- Chiara Jongerius
- Funding
-
 KWF, Unique High Risk project
KWF, Unique High Risk project
Immunotherapy has been a breakthrough in the treatment of cancer. Immunotherapy activates a person's own immune system to fight cancer. Checkpoint inhibition is the most commonly used immunotherapy for cancer and is approved to treat multiple types of malignancies. Patients with various cancer types benefit from immune checkpoint inhibition treatment.
Immune checkpoint inhibitors regulate T cell activity to promote T cell-mediated tumour cell death. However, checkpoint inhibition of T cells can also cause toxic side effects on the gastrointestinal tract, brain, thyroid, lungs, skin and more. Moreover, due to high costs of inhibitors, the use of checkpoint inhibition is often not cost effective, as compared to for instance chemotherapy. Therefore, efforts are needed to improve and support immunotherapy and enhance its effects.
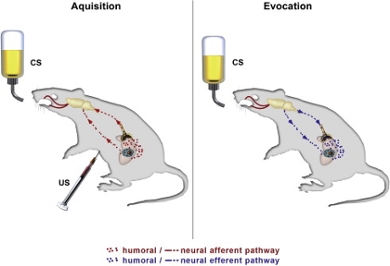
Evoking a conditioned immune reaction
We aim to test if immunotherapy can be supported by a conditioned immune response. A conditioned immune reaction could improve efficacy of treatment and decrease the high healthcare costs associated with immunotherapy. A conditioned immune reaction can be evoked by classical conditioning. The conditioning design uses a conditioned stimulus, which is an initially neutral stimulus (a taste or smell). Immune functions are stimulated by repeatedly pairing this conditioned stimulus to a medication that causes an immune reaction. When the conditioned stimulus is offered without the medication, the learned immune reaction still takes place. Next to this, the conditioning paradigm can be used to reduce medication doses, while improving or maintaining the treatment’s effectiveness. However, conditioning of the immune response after immune checkpoint inhibition in relation to cancer treatment has never been tested before, even though it may have widespread consequences for checkpoint inhibition therapy in different cancer types. Investigating the conditioning of immune checkpoint inhibition may also inform about immune mechanisms in cancer therapy that can influence future treatments. Therefore, it is important to investigate the effects and mechanisms of conditioning of immunotherapy for the treatment of cancer.
We hypothesize that the conditioning paradigm offers an effective strategy to improve efficacy of checkpoint inhibition immunotherapy and reducing its doses for cancer treatment. Therefore, we aim to test if conditioning, i.e. the repeated pairing of immune checkpoint inhibition with a conditioned stimulus, results in an altered immune response. This learned immune response mimics the effect of the medication. We thus hypothesize that the conditioned immune reaction will contribute to tumour growth reduction.
Murine cancer models
We aim to test if the conditioning paradigm is applicable to checkpoint inhibition in murine cancer models. We want to establish a proof of concept in murine models to demonstrate the biological mechanisms behind a standardized protocol.
With the proposed experimental design, we will be able to confirm or reject our key hypothesis. We expect that the conditioning paradigm improves the effects of immunotherapy, which will have wide implications for treatment of more cancer types. Conditioning has the potential to make immunotherapy more effective and safe in a simple and non-invasive way, by reducing medication doses and retaining treatment efficacy. To validate findings in humans, outcomes of our experiments will be translated in future clinical trials to be able to improve the treatment for cancer patients.
