Research project
Reactivity on interstellar ice analogues
The "empty" space between the stars is actually filled with a non-homogeneous mixture composed of roughly 99% gaseous species and 1% micron-sized dust grains. A plethora of molecules have been detected in the interstellar medium, ranging from simple di-atomic molecules, to complex ones, such as sugars, amides, and polycyclic aromatic hydrocarbons. These molecules carry the elements critical to life: C, H, O, N, P, and S.
- Contact
- Thanja Lamberts

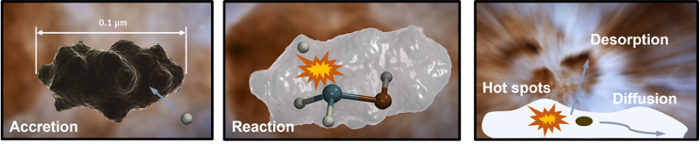
The star formation evolutionary cycle starts in dense, dark molecular clouds and these quiescent regions play a crucial role for interstellar chemistry. Gas-phase atoms and molecules accrete on top of dust grains, diffuse, react and eventually desorb (evaporate), see also Figure 1. Saturated species, such as water or methanol, are thought to originate from grain surface chemistry. Their formation is driven predominantly by radical-radical reactions and sequential hydrogenation via tunneling.
This way, an icy molecular mantle builds up over the course of roughly a million years. Such mantles are typically composed of amorphous material with thicknesses up to 100 monolayers. Water and carbon dioxide are the main ice components formed at early times, while, later, the presence of carbon monoxide leads to an apolar ice coating.
The James Webb Space Telescope, launched in December 2021, will soon shine light on the ice composition and detect new, possibly complex organic, species in situ in starless and pre-stellar cores. In the Lamberts group, we study surface processes on ice-covered dust grains that take place in the dense and cold (10 K) interstellar regions, where an intricate interplay between adsorption, diffusion, reaction and subsequent desorption into the gas phase takes place. The ultimate goal is to understand which (pre-biotic) molecules can be efficiently formed and link computational work to observational, experimental, and modeling studies at the Leiden Observatory.
An up-to-date overview of the publications of Thanja Lamberts can be found on Google Scholar.