Research project
Better ligands for G Protein-Coupled Receptors
The receptor nomenclature committee of IUPHAR, the International Union of Pharmacology, has several subgroups. Among these are a few that our division is involved in, those for adenosine, nicotinic acid, and GnRH receptors.
- Contact
- Adriaan IJzerman
That involvement is reflected in the web pages of the IUPHAR receptor database , and in reviews that appear in the high-impact journal ‘Pharmacological Reviews’.
Ad IJzerman and colleagues have written such reviews on e.g., adenosine receptors that have become citation classics. Pivotal to these reviews, is information that describes ligands that are used as pharmacological tool compounds. Despite the almost endless synthetic efforts, such ligands may not be that excellent in terms of affinity and/or selectivity for e.g., human receptors. Moreover, the binding kinetics of such ligands have rarely been characterized, let alone of being used for lead optimization. Also, the concept that ligands may occupy distinct sites on receptors (orthosteric vs allosteric binding) has not been fully explored. We have set ourselves the goal of synthesizing tailored compounds for the drug targets in our research programs, along four lines depending on the target’s ‘need’.
Affinity
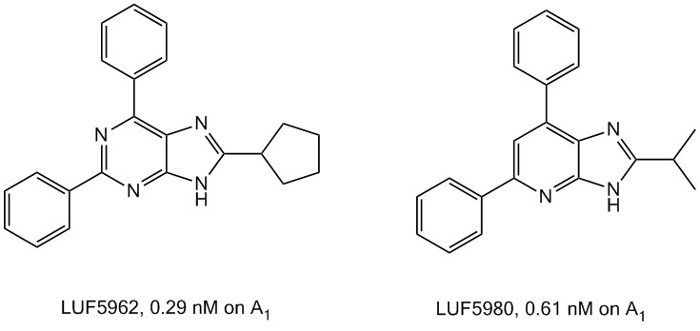
Designing and synthesizing high-affinity ligands for a given drug target have always been among the more important challenges in the drug discovery process. We have done that routinely over the years and have been successful on many occasions. It does make sense to have high affinity, next to other design criteria. Administering, as a consequence, low amounts of compound to a patient is often beneficial in terms of side effects, and particularly in metabolic load. Interestingly, compounds with super high affinity (that is subnanomolar affinity) are rare, and we’re proud that we’ve managed to develop a few for the adenosine receptors, such as LUF5962 and LUF5980.
Publications
Guo D, Xia L, van Veldhoven JP, Hazeu M, Mocking T, Brussee J, Ijzerman AP, Heitman LH. Binding kinetics of ZM241385 derivatives at the human adenosine A2A receptor. ChemMedChem. 2014 Apr;9(4):752-61.
Chang LC, von Frijtag Drabbe Künzel JK, Mulder-Krieger T, Westerhout J, Spangenberg T, Brussee J, Ijzerman AP. 2,6,8-trisubstituted 1-deazapurines as adenosine receptor antagonists. J Med Chem. 2007 Feb 22;50(4):828-34.
Chang LC, Spanjersberg RF, von Frijtag Drabbe Künzel JK, Mulder-Krieger T, Brussee J, Ijzerman AP. 2,6-disubstituted and 2,6,8-trisubstituted purines as adenosine receptor antagonists. J Med Chem. 2006 May 18;49(10):2861-7.
Selectivity
Target selectivity is an important aspect of any drug molecule, and certainly a parameter to be optimized. That is not trivial for a number of reasons. First of all hundreds of drug targets (receptors, enzymes, ion channels) exist, and no single lab in the world has assays for all of them. So, routinely, ligands tend to be tested against a small number of related targets, such as the four subtypes of adenosine receptors. Secondly, selectivity often is species-dependent. Particularly, compounds from the past, developed in animal studies, may have good selectivity in e.g. rodent tissue, but fail to demonstrate such selectivity in human tissues. Vice versa, compounds profiled in humanized assays, e.g. CHO cells overexpressing a human receptor, may face difficulties in further development in advanced animal models of disease.
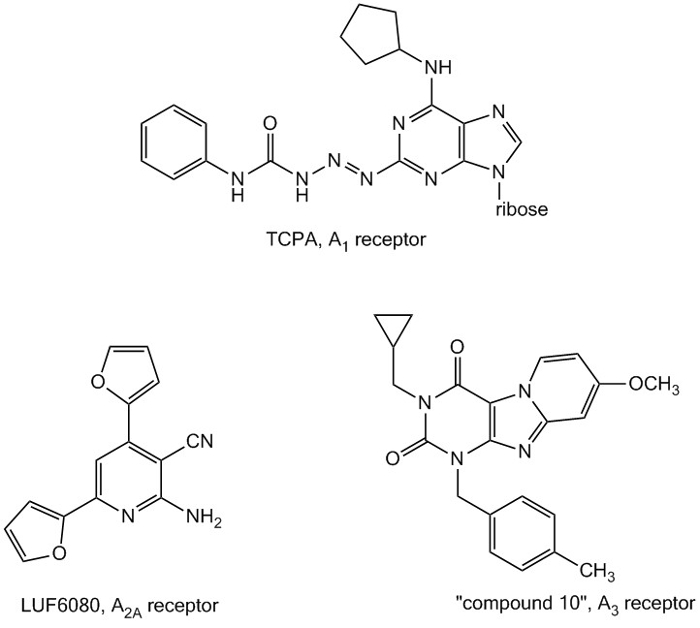
We have focused on the synthesis of truly selective ligands for human adenosine receptors, and identified TCPA as a highly selective agonist for the human adenosine A1 receptor, LUF6080 with reasonable selectivity as an antagonist for the adenosine A2A receptor and ‘compound 10’, a highly selective antagonist for the adenosine A3 receptor.
We are now very interested in the concept of ‘kinetic selectivity’. Compounds may have different kinetic profiles of interaction at related drug targets. Tiotropium is a nice example – it has almost equal affinity for all five muscarinic receptor subtypes, but it sits on the M3 receptor for a very long time, unlike the other four. In practice, this means that tiotropium is quite selective in its actions in vivo, being used in the treatment of COPD. We have recently explored this phenomenon ourselves for the adenosine and tachykinin (NK1) receptors.
Last but not least, we pay significant attention to the computational prediction of selectivity. This is by no means trivial, but the first steps have been set. We’re helped here by the high-resolution crystal structures of e.g. adenosine receptors, a research area in which we are actively involved.
Publications
Beukers MW, Wanner MJ, Von Frijtag Drabbe Künzel JK, Klaasse EC, IJzerman AP, Koomen GJ. N6-cyclopentyl-2-(3-phenylaminocarbonyltriazene-1-yl)adenosine (TCPA), a very selective agonist with high affinity for the human adenosine A1 receptor. J Med Chem. 2003 Apr 10;46(8):1492-503.
Mantri M, de Graaf O, van Veldhoven J, Göblyös A, von Frijtag Drabbe Künzel JK, Mulder-Krieger T, Link R, de Vries H, Beukers MW, Brussee J, Ijzerman AP. 2-Amino-6-furan-2-yl-4-substituted nicotinonitriles as A2A adenosine receptor antagonists. J Med Chem. 2008 Aug 14;51(15):4449-55.
Priego EM, Pérez-Pérez MJ, von Frijtag Drabbe Kuenzel JK, de Vries H, Ijzerman AP, Camarasa MJ, Martín-Santamaría S. Selective human adenosine A3 antagonists based on pyrido[2,1-f]purine-2,4-diones: novel features of hA3 antagonist binding. ChemMedChem. 2008 Jan;3(1):111-9.
Nederpelt I, Bleeker D, Tuijt B, IJzerman AP, Heitman LH. Kinetic binding and activation profiles of endogenous tachykinins targeting the NK1 receptor. Biochem Pharmacol. 2016 Aug 5.
Guo D, Dijksteel GS, van Duijl T, Heezen M, Heitman LH, IJzerman AP. Equilibrium and kinetic selectivity profiling on the human adenosine receptors. Biochem Pharmacol. 2016 Apr 1;105:34-41.
van Westen GJ, van den Hoven OO, van der Pijl R, Mulder-Krieger T, de Vries H, Wegner JK, Ijzerman AP, van Vlijmen HW, Bender A. Identifying novel adenosine receptor ligands by simultaneous proteochemometric modeling of rat and human bioactivity data. J Med Chem. 2012 Aug 23;55(16):7010-20.
Sanders MP, Roumen L, van der Horst E, Lane JR, Vischer HF, van Offenbeek J, de Vries H, Verhoeven S, Chow KY, Verkaar F, Beukers MW, McGuire R, Leurs R, Ijzerman AP, de Vlieg J, de Esch IJ, Zaman GJ, Klomp JP, Bender A, de Graaf C. A prospective cross-screening study on G-protein-coupled receptors: lessons learned in virtual compound library design. J Med Chem. 2012 Jun 14;55(11):5311-25.
Katritch V, Jaakola VP, Lane JR, Lin J, Ijzerman AP, Yeager M, Kufareva I, Stevens RC, Abagyan R. Structure-based discovery of novel chemotypes for adenosine A(2A) receptor antagonists. J Med Chem. 2010 Feb 25;53(4):1799-809.
Binding kinetics
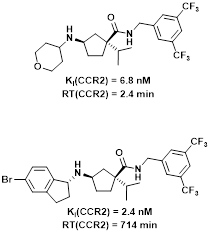
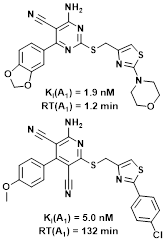
A concept that has emerged in our recent research is the one of binding kinetics. Binding kinetics is notably embodied through residence time (RT), a direct reflection of how long a drug stays bound to its target. This parameter is of crucial importance, because drug action lasts only as long as the receptor-ligand complex exists. Interesting results in the new field of Structure-Kinetics Relationships (SKR) have been obtained with quite varied targets, such as the CCR2, GnRH, HCA2, A1 and A2A receptors. In these studies, we observed that small changes to the structure of a ligand can bring about changes in residence time of up to two orders of magnitude (from one minute to more than 10 hours).
Publications
Nederpelt I, Georgi V, Schiele F, Nowak-Reppel K, Fernández-Montalván AE, IJzerman AP, Heitman LH. Characterization of 12 GnRH peptide agonists - a kinetic perspective. Br J Pharmacol. 2016 Jan;173(1):128-41.
Louvel J, Guo D, Soethoudt M, Mocking TA, Lenselink EB, Mulder-Krieger T, Heitman LH, IJzerman AP. Structure-kinetics relationships of Capadenoson derivatives as adenosine A1 receptor agonists. Eur J Med Chem. 2015 Aug 28;101:681-91.
Yu Z, van Veldhoven JP, Louvel J, 't Hart IM, Rook MB, van der Heyden MA, Heitman LH, IJzerman AP. Structure-Affinity Relationships (SARs) and Structure-Kinetics Relationships (SKRs) of Kv11.1 Blockers. J Med Chem. 2015 Aug 13;58(15):5916-29.
Vilums M, Zweemer AJ, Dilanchian A, van Veldhoven JP, de Vries H, Brussee J, Saunders J, Stamos D, Heitman LH, IJzerman AP. Evaluation of (4-Arylpiperidin-1-yl)cyclopentanecarboxamides As High-Affinity and Long-Residence-Time Antagonists for the CCR2 Receptor. ChemMedChem. 2015 Jul;10(7):1249-58.
van Veldhoven JP, Liu R, Thee SA, Wouters Y, Verhoork SJ, Mooiman C, Louvel J, IJzerman AP. Affinity and kinetics study of anthranilic acids as HCA2 receptor agonists. Bioorg Med Chem. 2015 Jul 15;23(14):4013-25.
Vilums M, Zweemer AJ, Barmare F, van der Gracht AM, Bleeker DC, Yu Z, de Vries H, Gross R, Clemens J, Krenitsky P, Brussee J, Stamos D, Saunders J, Heitman LH, IJzerman AP. When structure-affinity relationships meet structure-kinetics relationships: 3-((Inden-1-yl)amino)-1-isopropyl-cyclopentane-1-carboxamides as CCR2 antagonists. Eur J Med Chem. 2015 Mar 26;93:121-34.
Louvel J, Guo D, Agliardi M, Mocking TA, Kars R, Pham TP, Xia L, de Vries H, Brussee J, Heitman LH, Ijzerman AP. Agonists for the adenosine A1 receptor with tunable residence time. A Case for nonribose 4-amino-6-aryl-5-cyano-2-thiopyrimidines. J Med Chem. 2014 Apr 24;57(8):3213-22.
Vilums M, Zweemer AJ, Yu Z, de Vries H, Hillger JM, Wapenaar H, Bollen IA, Barmare F, Gross R, Clemens J, Krenitsky P, Brussee J, Stamos D, Saunders J, Heitman LH, Ijzerman AP. Structure-kinetic relationships--an overlooked parameter in hit-to-lead optimization: a case of cyclopentylamines as chemokine receptor 2 antagonists. J Med Chem. 2013 Oct 10;56(19):7706-14.
We’re often asked to comment on the subject of binding kinetics, which has led to a number of viewpoint papers and reviews.
Publications
Guo D, Heitman LH, IJzerman AP. Kinetic Aspects of the Interaction between Ligand and G Protein-Coupled Receptor: The Case of the Adenosine Receptors. Chem Rev. 2016 Apr 18.
Guo D, Heitman LH, IJzerman AP. The Role of Target Binding Kinetics in Drug Discovery. ChemMedChem. 2015 Nov;10:1793-6.
Allosterism
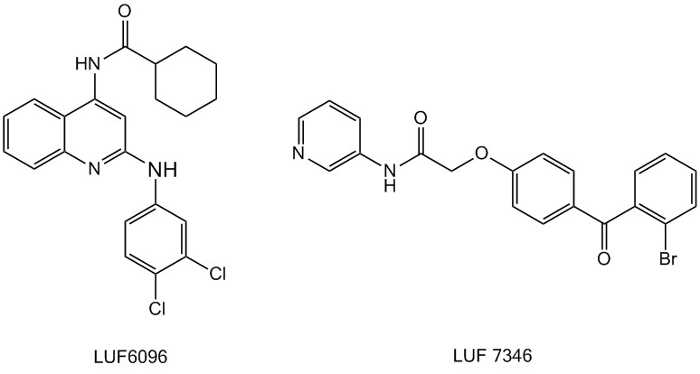
The recognition that there may be more, so-called allosteric binding sites on a given receptor has also fuelled our synthetic efforts. Over the last few years we have focused on many drug targets (adenosine A1, A2A and A3 receptors, the mGlu2 receptor and a classic ‘anti-target’, the hERG channel) to explore this notion. We have identified and designed new compounds that enhance the action of a classic agonist (a so-called positive allosteric modulator – PAM) or, very differently, act as a new class of antagonists (negative allosteric modulators – NAMs). As a PAM for the adenosine A3 receptor we developed the promising LUF 6096. A NAM we have developed for the hERG channel (exemplified by LUF 7346) may even reverse the cardiotoxicity induced by hERG somatic mutations, and save the life of patients suffering from this genetic malfunctioning. Such compounds would be fantastic representatives of true ‘precision medicine’.
Publications
Sala L, Yu Z, Ward-van Oostwaard D, van Veldhoven JP, Moretti A, Laugwitz KL, Mummery CL, IJzerman AP, Bellin M. A new hERG allosteric modulator rescues genetic and drug-induced long-QT syndrome phenotypes in cardiomyocytes from isogenic pairs of patient induced pluripotent stem cells. EMBO Mol Med. 2016 Jul 28. pii: e201606260.
Massink A, Louvel J, Adlere I, van Veen C, Huisman BJ, Dijksteel GS, Guo D, Lenselink EB, Buckley BJ, Matthews H, Ranson M, Kelso M, IJzerman AP. 5'-Substituted Amiloride Derivatives as Allosteric Modulators Binding in the Sodium Ion Pocket of the Adenosine A2A Receptor. J Med Chem. 2016 May 26;59(10):4769-77.
Yu Z, Liu J, van Veldhoven JP, IJzerman AP, Schalij MJ, Pijnappels DA, Heitman LH, de Vries AA. Allosteric Modulation of Kv11.1 (hERG) Channels Protects Against Drug-Induced Ventricular Arrhythmias. Circ Arrhythm Electrophysiol. 2016 Apr;9(4):e003439.
Doornbos ML, Pérez-Benito L, Tresadern G, Mulder-Krieger T, Biesmans I, Trabanco AA, Cid JM, Lavreysen H, IJzerman AP, Heitman LH. Molecular mechanism of positive allosteric modulation of the metabotropic glutamate receptor 2 by JNJ-46281222. Br J Pharmacol. 2016 Feb;173(3):588-600.
Yu Z, van Veldhoven JP, 't Hart IM, Kopf AH, Heitman LH, IJzerman AP. Synthesis and biological evaluation of negative allosteric modulators of the Kv11.1(hERG) channel. Eur J Med Chem. 2015 Dec 1;106:50-9.
Blad CC, van Veldhoven JP, Klopman C, Wolfram DR, Brussee J, Lane JR, Ijzerman AP. Novel 3,6,7-substituted pyrazolopyrimidines as positive allosteric modulators for the hydroxycarboxylic acid receptor 2 (GPR109A). J Med Chem. 2012 Apr 12;55(7):3563-7.
Du L, Gao ZG, Nithipatikom K, Ijzerman AP, Veldhoven JP, Jacobson KA, Gross GJ, Auchampach JA. Protection from myocardial ischemia/reperfusion injury by a positive allosteric modulator of the A₃ adenosine receptor. J Pharmacol Exp Ther. 2012 Jan;340(1):210-7.
- Fredholm et al. Pharmacol. Rev. 2001, 53, 1-26; Pharmacol. Rev. 2011, 63, 1-34.
- Vilums, Zweemer et al. J. Med. Chem. 2013, 56, 7706-7714; Eur. J. Med. Chem. 2015, 93, 121-134.
- Nederpelt et al. Br. J. Pharmacol. 2015, in press
- Liu, van Veldhoven et al. Bioorg. Med. Chem. 2015, 23, 4013-4025.
- Louvel, Guo et al. J. Med. Chem. 2014, 57, 3213-3222; Eur. J. Med. Chem. 2015, 101, 681-691
- Guo, Xia et al. ChemMedChem 2014, 9, 752-761.
- Heitman, Göblyös et al., J. Med. Chem. 2009, 52, 926-931; Du et al. J. Pharm. Exp. Ther. 2012, 340, 210-217.
- Blad, van Veldhoven et al. J. Med. Chem. 2012, 55, 3563-3567.
