Research project
Allosteric Modulation
Allosteric modulation has long been recognized as a general and widespread mechanism for the control of protein function. Modulators bind to regulatory sites distinct from the active site on the protein, resulting in conformational changes that may profoundly influence protein function. This concept has been studied intensively in the past decades and is now considered a classical control mechanism of protein behaviour.
- Contact
- Laura Heitman
Interestingly, it is increasingly realized that G protein-coupled receptors (GPCRs) may also be subject to allosteric modulation. As mentioned before, many researchers thought that drugs are either agonists or antagonists, occupying, at least partly, the same ligand binding site on the receptor. This "primary" binding site is also thought to accommodate endogenous ligands such as hormones and neurotransmitters, adenosine for instance. However, the interaction of e.g., an agonist with its receptor often results in a continuous stimulation of potentially all receptor molecules in the body, which is not necessarily desirable. Similarly, the interaction of an antagonist with a receptor may lead to a prolonged blockade of receptor function, which may not be compatible with the "kinetics" of a pathological condition.
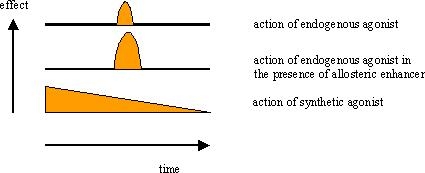
Allosteric modulation is a far more natural way of mimicking the actions of the body's own agonists, such as hormones and neurotransmitters; without the endogenous agonist the allosteric modulator is without effect.
There may, however, be novel ways to intercede in receptor function that allow for a more controlled and selective "tuning" action on the receptor. This is inherent in ligands which bind at a second (allosteric) site and modify the binding and actions of the endogenous signalling molecule at the primary binding site. This offers an additional advantage for GPCRs that have peptides or proteins as endogenous ligands (e.g. GnRH and LH). Namely, the allosteric site can be targeted with low-molecular-weight (LMW) ligands that have the potential for oral bioavailability.
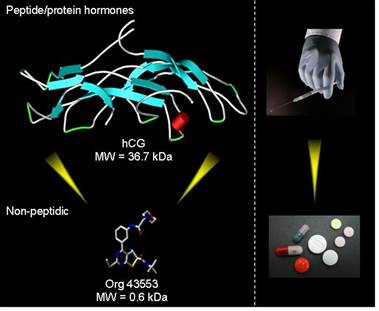
Oral bioavailability is one of the advantages of low-molecular-weight (allosteric) ligands
We have explored the action of so-called allosteric enhancers for the adenosine receptors. The idea is that an allosteric enhancer alone is not active, but only in combination with adenosine, the endogenous local hormone. In the late eighties of the previous century Fred Bruns (then at ParkeDavis) discovered such compounds for the adenosine A 1 receptor, such as PD81,723, a selective enhancer for this receptor. We have contributed to this field by synthesizing more potent PD81,723 analogs. More recently we discovered (selective) allosteric enhancers for the adenosine A 3 receptor as well, such as LUF6000 and LUF6096. In addition, we have investigated whether allosteric modulation is a general phenomenon on GPCRs. Therefore, we also looked at the GnRH and LH receptors, which are both class A GPCRs like the adenosine receptors. It appeared that these receptors can be allosterically modulated by LMW ligands, such as LUF5419 and LUF5771. This may provide another opportunity for the discovery of new orally bioavailable ligands. In addition, an extension of these studies showed that both receptors contain not just one, but two allosteric sites.
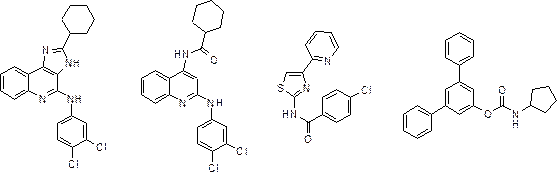
Novel allosteric modulators (from left to right): LUF6000, LUF6096, LUF5419 and LUF5771
If you want to know more about this exciting subject, read our recent (review) articles:
Zweemer AJ, Bunnik J, Veenhuizen M, Miraglia F, Lenselink EB, Vilums M, de Vries H, Gibert A, Thiele S, Rosenkilde MM, IJzerman AP, Heitman LH. Discoveryand Mapping of an Intracellular Antagonist Binding Site at the Chemokine Receptor CCR2. Mol Pharmacol. 2014, 86(4):358-68.
Yu Z, Klaasse E, Heitman LH, IJzerman AP. Allosteric modulators of the hERG K(+) channel: radioligand binding assays reveal allosteric characteristics of dofetilide analogs. Toxicol Appl Pharmacol. 2014, 274(1):78-86.
Gutiérrez-de-Terán H, Massink A, Rodríguez D, Liu W, Han GW, Joseph JS, Katritch I, Heitman LH, Xia L, IJzerman AP, Cherezov V, Katritch V, Stevens RC. The role of a sodium ion binding site in the allosteric modulation of the A(2A)adenosine G protein-coupled receptor. Structure 2013, 21(12):2175-85.
Zweemer AJ, Nederpelt I, Vrieling H, Hafith S, Doornbos ML, de Vries H, Abt J, Gross R, Stamos D, Saunders J, Smit MJ, IJzerman AP, Heitman LH. Multiple binding sites for small-molecule antagonists at the CC chemokine receptor 2. Mol Pharmacol. 2013 84(4):551-61.
Liu W, Chun E, Thompson AA, Chubukov P, Xu F, Katritch V, Han GW, Roth CB, Heitman LH, IJzerman AP, Cherezov V, Stevens RC. Structural basis for allosteric regulation of GPCRs by sodium ions. Science 2012, 337(6091):232-6.
Heitman LH, Kleinau G, Brussee J, Krause G, IJzerman AP. Determination of different putative allosteric binding pockets at the lutropin receptor by using diverse drug-like low molecular weight ligands. Mol Cell Endocrinol. 2012, 351(2):326-36.
Göblyös A, IJzerman AP. Allosteric modulation of adenosine receptors. Biochim Biophys Acta. 2011, 1808(5):1309-18.
