Research project
Glucocorticoids in zebrafish
A novel in vivo model system for studies on glucocorticoid resistance
- Contact
- Marcel Schaaf
- Funding
-
 SmartMix program of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science
SmartMix program of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science
Glucocorticoids regulate a wide range of systems in vertebrate organisms, and their effects are mediated by the glucocorticoid receptor (GR). The responsiveness to glucocorticoids differs largely between individuals. Resistance to glucocorticoids is an important medical problem, since it limits the efficacy of glucocorticoids when they are used to treat immune-related diseases like asthma and rheumatoid arthritis. Glucocorticoid resistance also contributes to the pathogenesis of other diseases, like major depression because of the decreased negative feedback on the hypothalamic pituitary adrenal axis. In this project, we use the zebrafish as an in vivo model system to study glucocorticoid resistance. First, the zebrafish is the only non-primate animal model in which a b-isoform of GR occurs, which is a splice variant with dominant-negative activity. Zebrafish are easily genetically modified, so the expression of GRb can be varied, creating an in vivo model for GRb-induced glucocorticoid resistance. Second, by performing a forward-genetic screen using the glucocorticoid-induced decrease in POMC expression in the pituitary gland as a readout, several zebrafish mutants have been obtained which appear to be resistant to glucocorticoid treatment. Thus, we use two types of in vivo models for studying the molecular mechanism of glucocorticoid signaling and resistance.
The first part of this project is carried out by a PhD student, Antonia Chatzopoulou. In order to study the function of zGRb in vivo, she will vary the expression of this GR isoform using several methods of genetic manipulation of zebrafish. First, using a morpholino that blocks the splice donor site in exon 8 (between exon 8 and 8b), the synthesis of zGRb mRNA can be increased. Since zGRa and zGRb mRNA are generated from the same pre-mRNA molecules, increased expression of the b-isoform coincides with a decreased zGRb expression in this approach (unpublished observation). Second, using microinjection of in vitro transcribed zGRb mRNA in early stage embryos (1-2 cell stage) zGRb is transiently overexpressed. Third, a transgenic fish line is currently being generated in our laboratory with inducible expression of zGRb. For this purpose, we are using the Gal4/UAS system. In this approach, the yeast transcription factor Gal4 is controlled by a heat-shock-inducible (HSP70) promoter, and zGRb expression is driven by a Gal4 recognition sequence (UAS). The function of zGRcan subsequently be determined in these genetically manipulated animal models. As a readout the expression level of glucocorticoid-responsive genes can be determined, e.g. by qPCR. Alternatively, using custom-made zebrafish microarrays or RNA sequencing, a more general view of the effects of zGRb on (glucocorticoid-induced changes in) gene expression will be obtained.
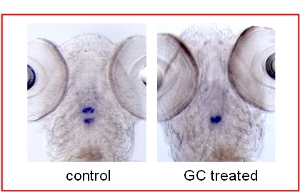
In the second part of the project, carried out by a postdoctoral fellow, Dr. Peter Schoonheim, the zebrafish is used as a model system to study glucocorticoid action during its feedback action on the hypothalamic pituitary interrenal (HPI) axis. Incubation of zebrafish larvae with glucocorticoids abolishes the expression of pomc in the corticotrope cells of the anterior lobe of the pituitary. This has been demonstrated by pomc mRNA in situ hybridization. Wehave recently performed a forward-genetic screen searching for zebrafish mutants that lack or have reduced glucocorticoid-induced suppression of pomc expression in the anterior lobe of the pituitary. In our relatively small pilot screen (300 F2 families), we have identified 10 zebrafish mutants that show altered expression of pomc in the presence of exogenously applied glucocorticoids. Upon crossing heterozygous mutants from these 10 mutant families with each other, three of these mutants fail to complement each other and thus most likely result from mutations in the same gene. To identify the genetic mutation that confers these phenotypes we will be using simple sequence repeat polymorphism mapping. These genes will give us many novel insights into the mechanisms of glucocorticoid feedback in the HPI axis.