Making fuels from sunlight and CO2
Plants could be regarded as small chemical factories, which produce chemical substances via photosynthesis. If we can imitate photosynthesis in an artificial system, we can make clean fuels and materials out of sunlight and CO2. Huub de Groot is very close to designing a system of this kind.
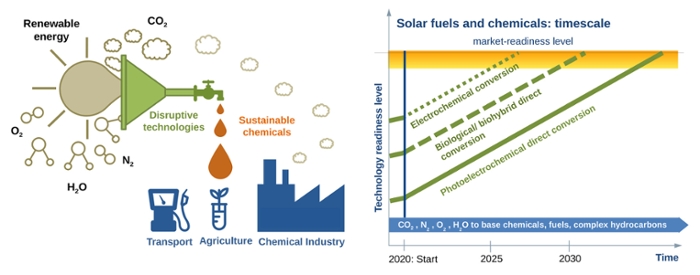
Solar energy is by far the earth’s most important energy source. By a process of photosynthesis, plants store the energy from sunlight in sugars, which they use to live and grow. Or to be more precise: plants take CO2 molecules out of the air, and capture sunlight with their chlorophyll; using this solar energy, they convert water and CO2 into oxygen and sugars. A plant is therefore a small chemical factory. Some time ago, scientists thought: if we can imitate the process of photosynthesis, we can extract CO2 from the air to make new products such as hydrogen, alcohol or ethylene with sunlight, without the use of oil or biomass. This is a completely clean, sustainable way to produce fuels and base materials.
Aiming for high efficiency
Huub de Groot’s research group has been conducting fundamental research since the 1990s in order to understand the process of photosynthesis down to the smallest details, before imitating and refining it. The latter is extremely important, because plants are far from efficient in their use of energy sources; they leave much of the captured light unused. De Groot is aiming for an artificial photosynthesis process in which 70% of the incoming light is both captured and used to make products. ‘If we succeed,’ says De Groot, ‘in Europe, we would be able to extract a maximum of 2,500 tonnes of CO2 per hectare per year from the air via artificial photosynthesis systems. This is a tremendously high conversion rate. And you would also be able to produce hundreds of tonnes of alcohol per hectare per year, for example.’
De Groot’s research group has succeeded in mapping the process of natural photosynthesis using magic-angle spinning NMR spectroscopy and computer simulations. An important step in elucidating photosynthesis was finding an organism with a chlorophyll antenna system that harvests light but does not contain proteins. Proteins are very often needed to optimise the chlorophyll for photosynthesis, but they are also coded by DNA. ‘So when you’re studying photosynthesis protein systems, you don’t know for sure whether you’re looking at an elementary, underlying physical-chemical principle or an effect of the DNA,’ De Groot explains. Plants contain proteins and were therefore not ideal as an object of study in which to learn about ‘pure’ photosynthesis. But back in the 1990s, the research group found another system: ‘chlorosomes.’ These are organelles that capture light and are found in green bacteria that live on the bed of lakes and seas. These bacteria also use photosynthesis to build molecules, but without protein.
Molecular dance
In this ‘protein-free’ environment, they observed that the molecules involved in photosynthesis engage in a kind of dance. ‘In preparation for photosynthesis, molecules start to vibrate and those vibrations cause the energy levels of the molecules to “cross” each other. When two energy levels in the same space cross each other, you get what are known as quantum instabilities. These cause energy transport and reactions to take place rapidly because the route from initial state to end product is very fast. The reaction is also never reversed. You create a kind of leak, a channel in the system, which makes the energy go exactly in the direction you want. This is the Goldilocks effect: the system itself finds the vibration that gives the best chance for the leak to transfer energy for a reaction.’ De Groot and a team of researchers from Groningen University and other countries also discovered that the chlorosomes offer a practical basis for designing artificial light antennae.
Mapping the molecular dance and discovering chlorosomes as the basis for an artificial system means that a giant step has been taken towards developing artificial photosynthesis. At the same time, however, some major things still need to happen before artificial systems are ready for use. For instance, parties have to be found that actually want to construct the systems. And for these to be interested, a system would have to be cheap enough to compete in the energy market. In addition, a solution still needs to be found for the surplus CO2 that you extract from the air with photosynthesis; how do you store it or use it?
The fundamental research is still ongoing. For instance, De Groot is engaged in discussions with a company about refining the computer models that portray the molecular dance. In the meantime, he is also taking steps to have the knowledge acquired converted into physical systems.

