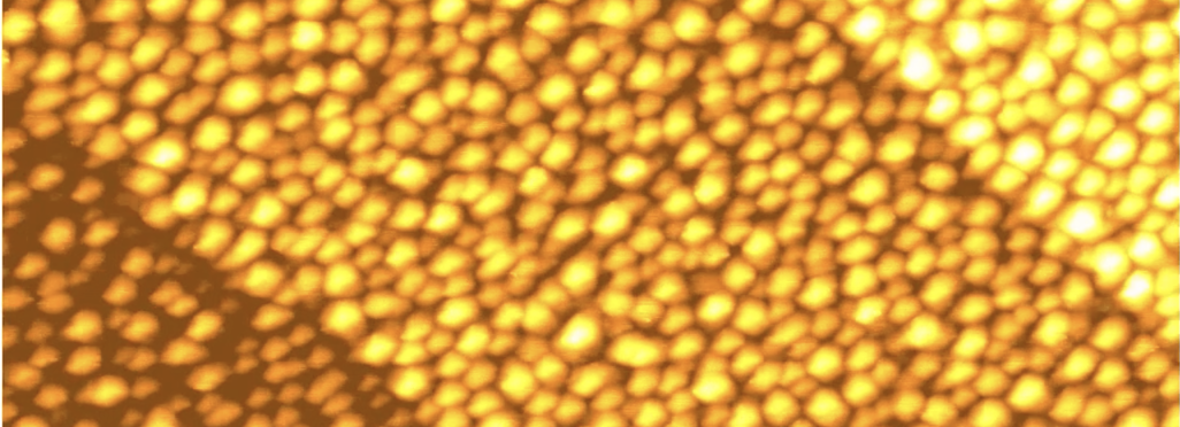
The roughening of a platinum electrode
Smooth platinum electrodes roughen and wear when subjected to repeated cycles of oxidation and reduction, which causes nanometer-scale mounds to grow. Leiden chemists Leon Jacobse and Marc Koper, together with physicist Marcel Rost, discovered the exact details, using a unique tunneling microscope.
Electrodes made of the noble metal platinum are used in electrolysis and fuel cells because they hardly deteriorate despite intensive use. However, they're not completely inert, and they do wear with use. 'It has been known for more than a century that they have to be prepared before use', says Marc Koper, an electrochemist at the Leiden Institute of Chemistry (LIC).
Before it works optimally, the electrode has to undergo a few cycles of oxidation and reduction. 'It was assumed that this cleans the electrode, but this is certainly not the only thing happening', says Koper. Chemical and physics research has shown how repeatedly oxidizing and reducing roughens the platinum, but the exact mechanism behind this process has always been somewhat of a mystery.
In an earlier paper, the three scientists showed that the roughening can be imaged to almost atomic resolution with a special Scanning Tunneling Microscope built by physicist Marcel Rost at the Leiden Institute of Physics (LION).
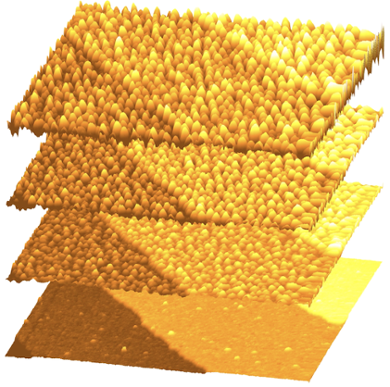
Platinum mounds
'An atomically sharp needle scans the surface, while we measure an extremely small current', says Rost. 'This is the so-called tunneling current that we use to image the surface atomically. But in this case, we can keep doing this while the surface and tip are part of an electrochemical cell, in which currents run many times larger than the tunneling current. This way, we can measure the reactivity while still imaging the surface.'
This technique makes visible how growing mounds form on the platinum surface. In the beginning, a perfect platinum surface is a plane of platinum atoms in a neat hexagonal lattice. When this surface oxidizes, a one atom thick layer of platinum oxide forms. In order to fit in the extra oxygen atoms, some platinum atoms are pushed out of the surface, and these atoms start to wander over the surface. These wandering atoms are called adatoms. During their travels, adatoms run into other adatoms, and they stick together to form small islands.
Surprising
When the platinum oxide layer is subsequently reduced, the adatom islands stay behind, along with the empty oxygen spots in the layer below, called vacancies. With repeated oxidizing and reducing, mounds start forming made out of stacked plateaus. The mounds become higher in the middle and deeper at the edges. 'This was surprising', says Rost, 'because mounds shouldn't be stable, and should merge together.'
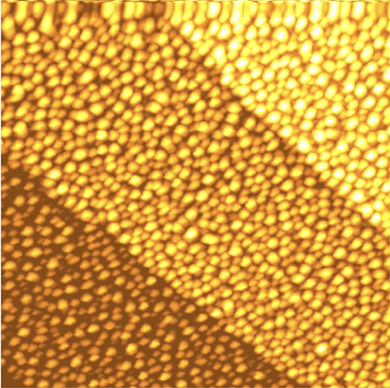
Holes
In their paper in ACS Central Science, the Leiden physics-chemistry cooperation maps out the mound evolution atom by atom. Starting out with an initially flat platinum surface in a solution of perchloric acid (HClO4) in water, they oxidized and reduced the platinum 170 times, by varying the electrical potential of the surface, while imaging the characteristic pockmarks caused by the mounds. By measuring the electrochemical current, and connecting that to the images, the researchers could specify the contributions of different steps and facets of the crystal plane.
But the exact shape and size of the mounds could only be explained after a conceptual leap. It is not only adatoms that can wander around, but vacancies can do the exact same thing. An atom next to the vacancy moves over to fill it, and thus the vacancy has moved by one atom. Similarly, vacancies can meet other vacancies to cling together. Just as adatoms form islands, vacancies can cling together to form growing holes.
Sustainable energy solutions
'The idea that a vacancy is a sort of anti-adatom is not new', says Rost, 'what is new is that both share similar growth modes to shape mounds and holes.' The mathematical description is identical.
With this insight, the theory of crystal growth (adding atoms) could be translated to the oxidation-reduction cycles (describing adatoms and vacancies simultaneously).
The growing holes and mounds, taken together, nicely explain the evolution of the mounds and thus the experimental roughness, the researchers show in a Nature Communications paper, that stresses the duality between adatoms and vacancies.
'Platina electrodes are used in electrochemical energy conversions, such as in electrolysis and fuel cells', says Koper. 'The wear and roughening of platinum electrodes is the most important factor in their longevity, and in the cost of new sustainable energy solutions. Now that we are gaining an atomically detailed understanding of this process, we can work in a much more focused way on improving these technologies.'
-
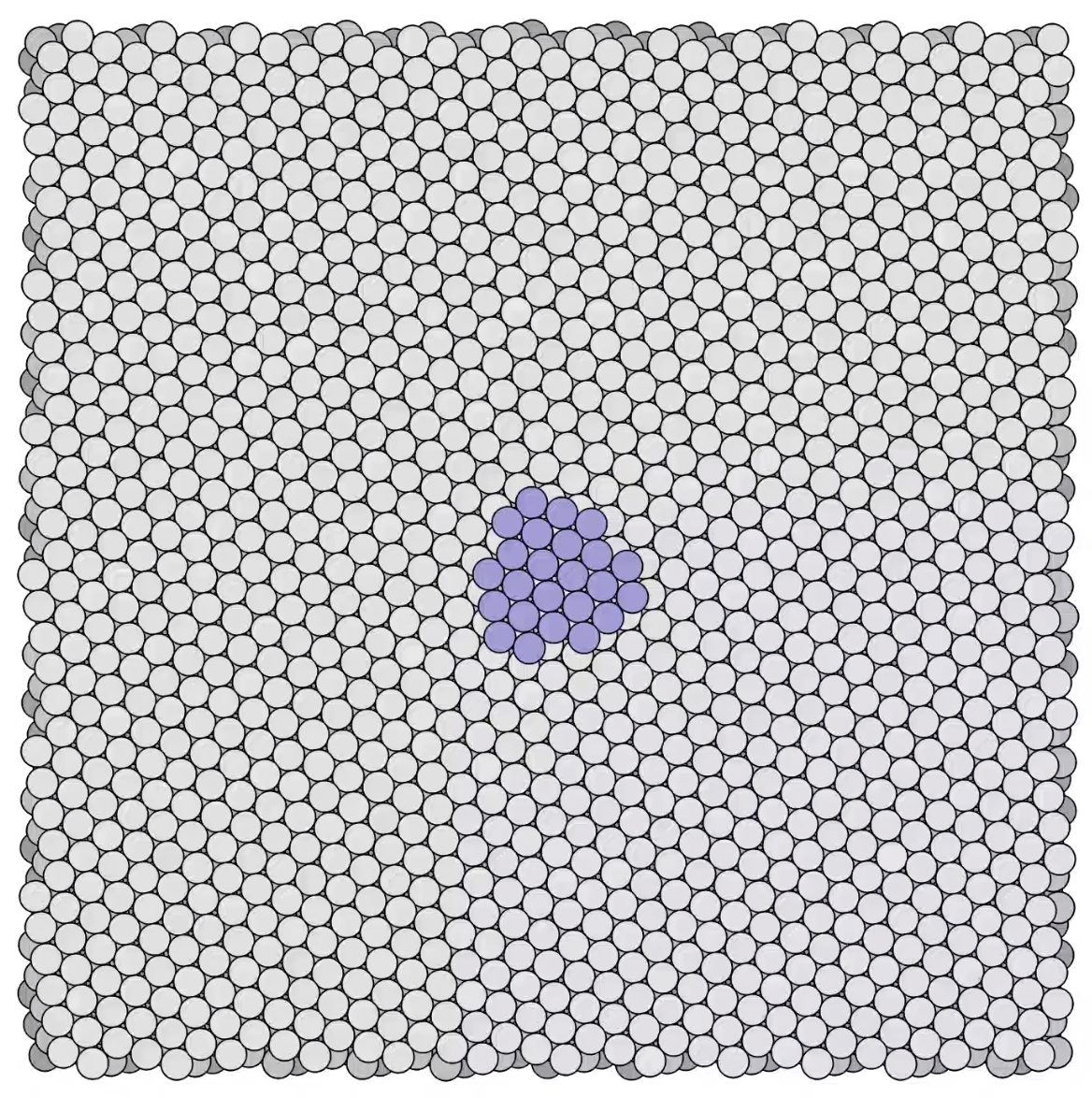
Stages in a growing platinum mound -
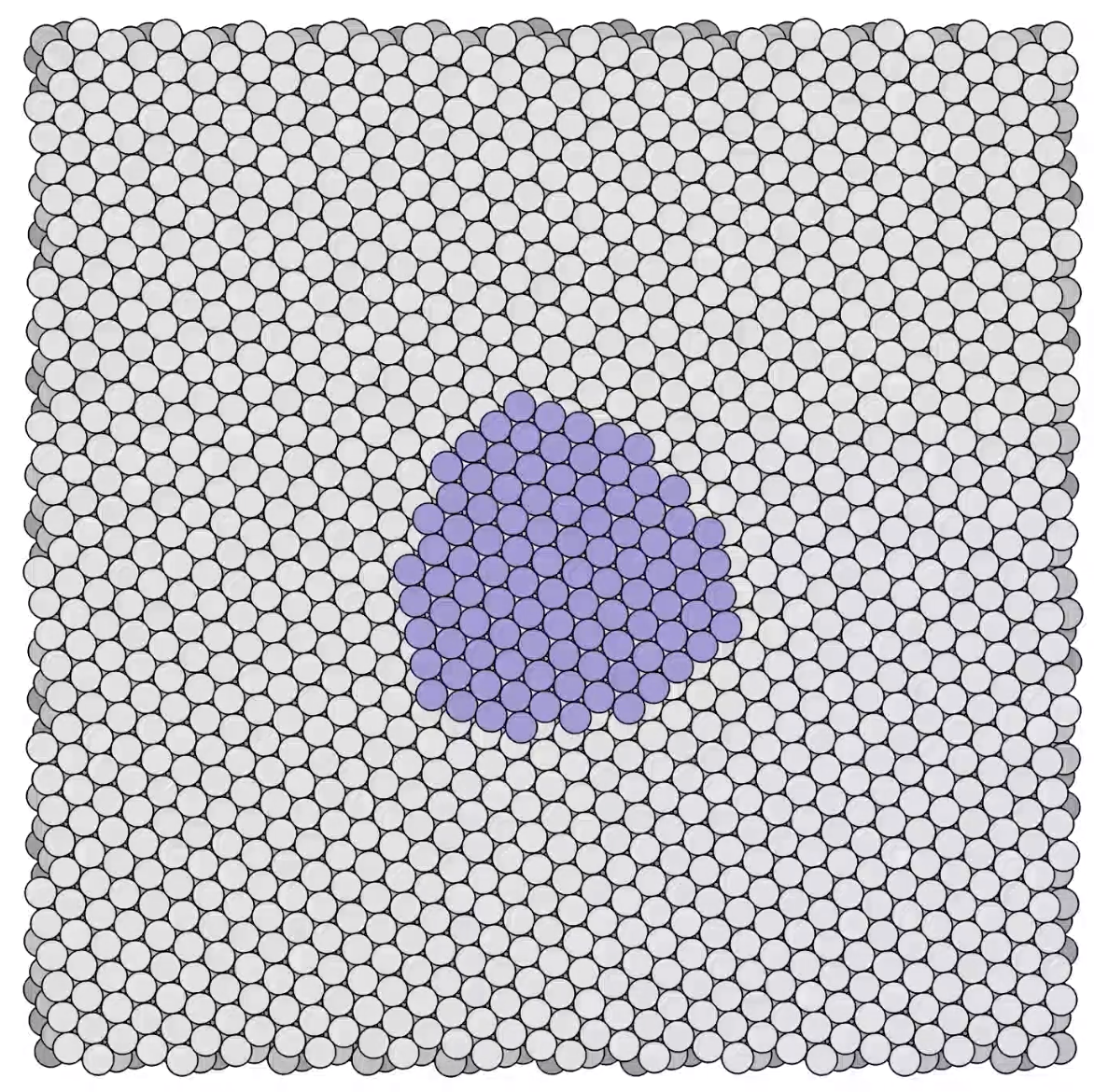
Stages in a growing platinum mound -
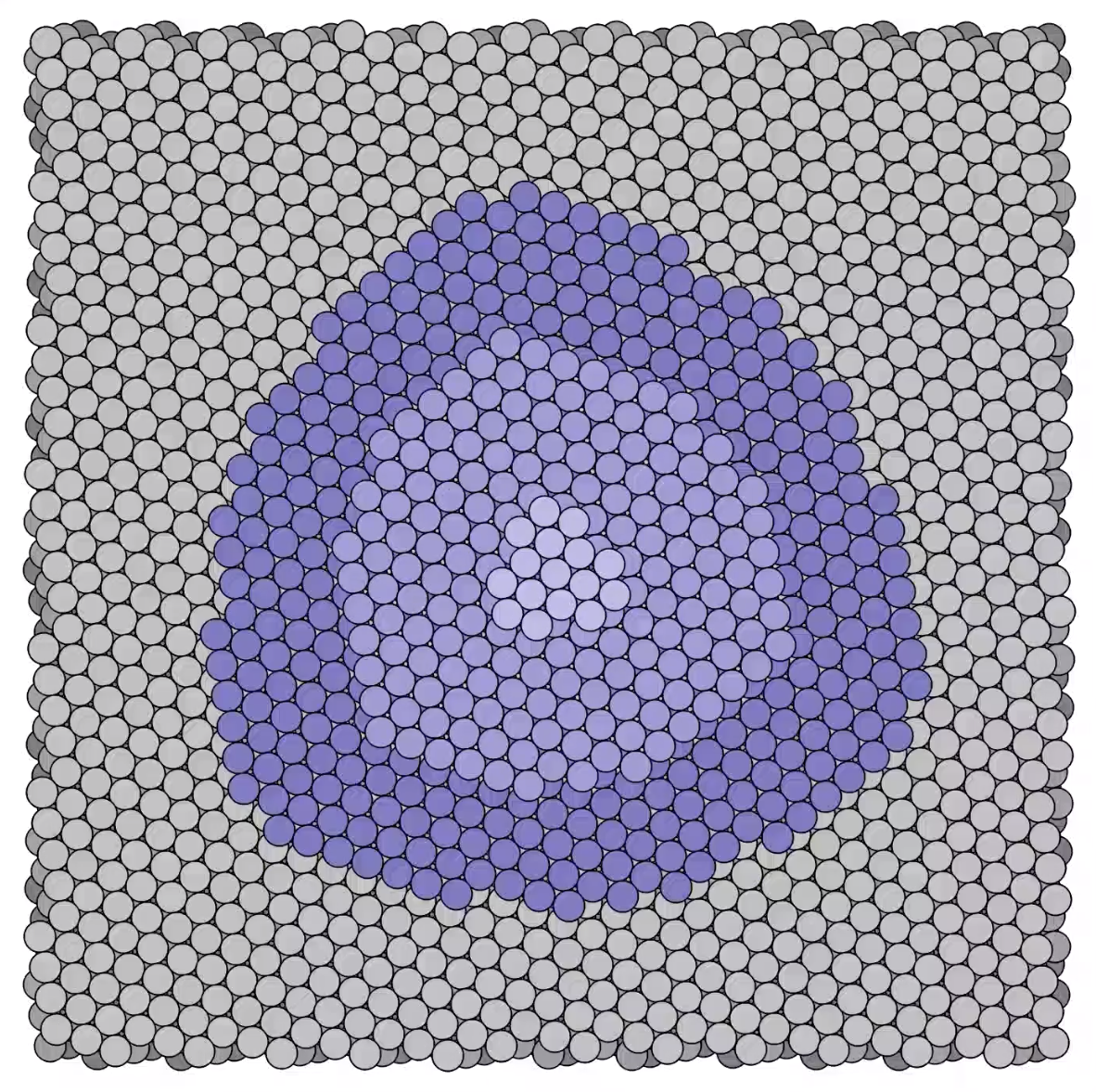
Stages in a growing platinum mound -
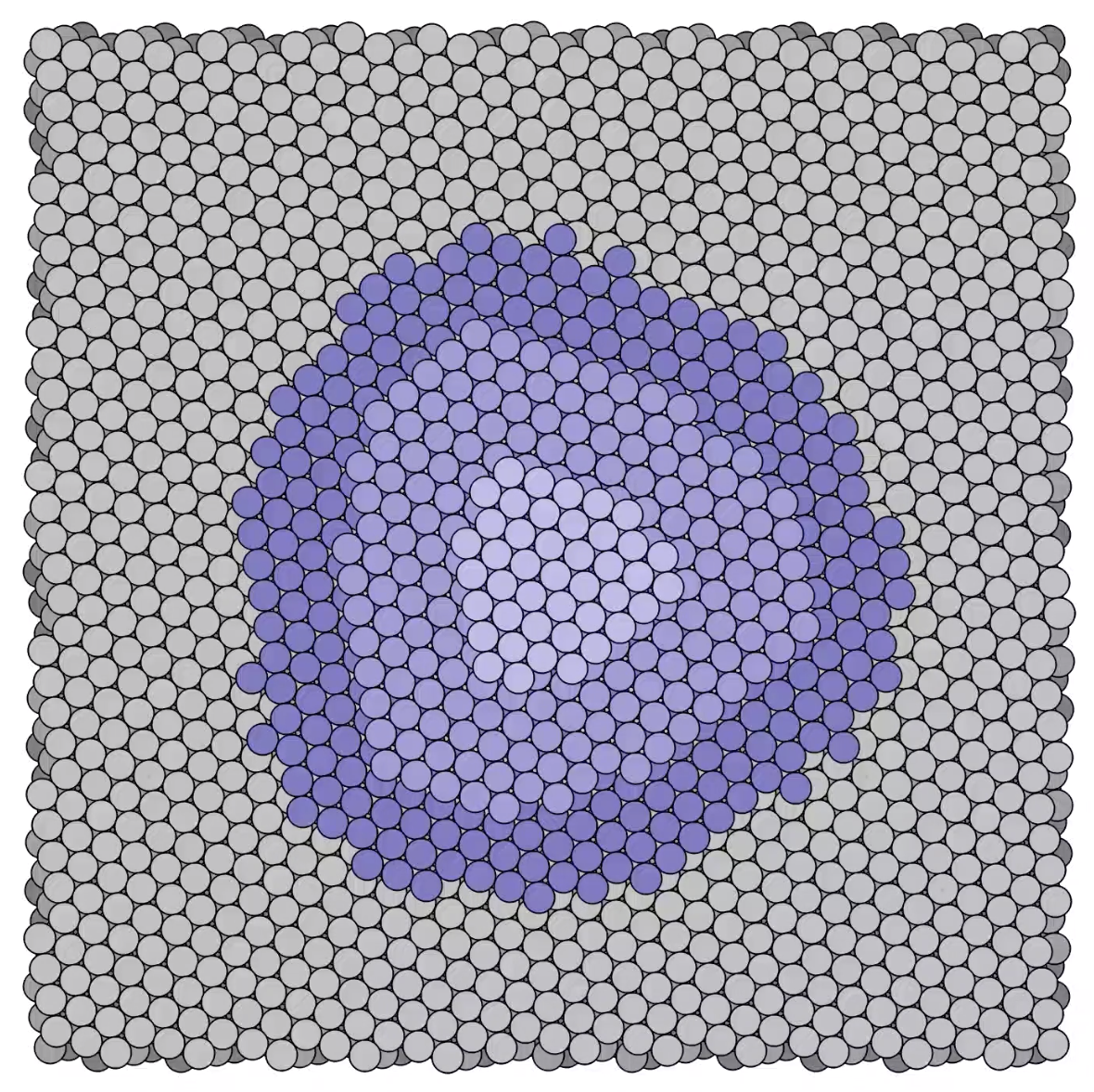
Stages in a growing platinum mound
Atomic-Scale Identification
Due to the selected cookie settings, we cannot show this video here.
Watch the video on the original website orThe Growth Dualism
Due to the selected cookie settings, we cannot show this video here.
Watch the video on the original website or