
Molecular mystery in space
The space between the stars isn't simply a void; it also contains substances such as methanol and carbon dioxide. So far, chemical theories have been unable to explain how these substances originate under circumstances that should make it impossible. Fedor Goumans has been awarded a Veni subsidy to try to resolve this mystery.
More rarified than air

Energy
Molecules in space are probably formed because of the dust particles floating around in particular areas to which loose atoms and molecules can attach themselves. The likelihood that these atoms meet with another atom on a dust paticle is therefore much greater than in the surrounding space. The dust particle can also absorb the energy that is released when a molecule is formed, so that the new molecule can cool down and stabilise. But this theory cannot explain the whole story.
Reaction barrier

‘We already know how carbon monoxide is generated, or at least we have a plausible explanation for it,' says Gouwmans. 'But there is a problem with carbon dioxide and methanol. A reaction barrier is needed to create these molecules.' This barrier is a kind of resistance that the atoms have to overcome before they can react with one another, rather like if a door is sticking and you have to use more strength to open it. Normally this barrier is overcome because the molecules move quickly (that is, if the temperature is high enough) and the collision generates enough energy for the reaction. But those regions in space where molecules form are too cold: the temperature is around 260 degrees below zero. This means that the atoms do not move quickly enough to overcome the reaction barrier.
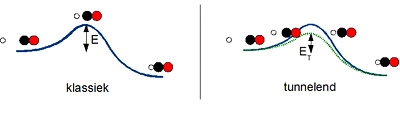
Tunnelling
How can these reactions take place, then? Goumans: ‘One possibility, or maybe the possibility is that the hydrogen atom tunnels through the barrier. When a particle 'tunnels', it is as if it is transported magically from one side of the obstacle to the other, without passing through the obstacle. You can compare it to if you were in your cellar and suddenly found yourself in the attic, without the cellar door opening or you having to climb the stairs. Hydrogen atoms are light enough to be able to tunnel a bit, and so are able to make a small step to reach the other side of the reaction barrier, which means the reaction takes place faster than you would expect. Maybe this tunnelling ensures that a hydrogen atom can attach itself to carbon monoxide as the first step in producing methanol.'
Space methanol
But scientists are still not able to explain it fully. Goumans: ‘You can check the calculations by looking at the ratio between normal hydrogen and heavy hydrogen atoms (deuterium), for example, in methanol in space. Hydrogen atoms are lighter than deuterium atoms, so they tunnel better, which means you would expect space methanol to contain relatively more hydrogen atoms and fewer deuterium atoms than the rest of space. But the opposite is true; so there must be other reactions that play a role.' The dust particle on which the reactions take place could also have an effect. Goumans is hoping that a forthcoming visit to Germany will be an opportunity to study how simulating the reactions can be linked to simulation of a dust particle.
Fosterite
There's obviously enough to do, but what drives Goumans? ‘What I like about this research is that I find things I hadn't expected; you think you've found something, but in fact it turns out to be something completely different. During my research in London, for example, I studied the mineral fosterite, and our calculations showed that when hydrogen bonded to fosterite, it divided; the nucleus bonded to an oxygen atom and the electron to the magnesium ion. So we then studied a similar mineral (magnesium oxide), and this strange mechanism was proven to exist. If you spend time making calculations and the results prove more or less what you had expected, it's a bit boring, don't you think?'
