Lecture
LIC Lecture: Helical supramolecular polymers - Toward structure-function relationships
- Date
- Monday 10 October 2022
- Time
- Location
-
Gorlaeus Building
Einsteinweg 55
2333 CC Leiden - Room
- DM1.15

Biography
Luis Sánchez was born in 1970 in Toledo (Spain) and is Full Professor of Organic Chemistry at the University Complutense of Madrid (UCM), Spain. He received his Ph.D. in Organic Chemistry at the University Complutense of Madrid in 1997 under the supervision of Prof. Carlos Seoane and Nazario Martín, where he carried out the synthesis and studied the properties of C60–donor dyads and triads.
From 1999 to 2000 he worked as a postdoctoral researcher with Professor Jan C. (Kees) Hummelen (University of Groningen, The Netherlands) on the synthesis of supramolecular architectures based on C60 and their application in the preparation of organic solar cells. In 2002, he was appointed as an associate professor at UCM and he has been promoted to Full Professor in October, 2017.
He is currently co-author of more than 125 publications and was awarded with the Prize to Novel Researchers of the RSEQ in 2003. He has directed and co-directed seven Doctoral Theses and four more that are currently underway. In 2020, he was awarded with the Ignacio Ribas Medal by the Specialized Group on Organic Chemistry of the Spanish Royal Society of Chemistry (RSEQ).
Abstract
A challenging goal in the field of supramolecular polymers is stablishing clear structure-function relationships. These rules could help to predict and account for outstanding properties of the supramolecular polymers formed by the self-assembly of a number of monomeric species.
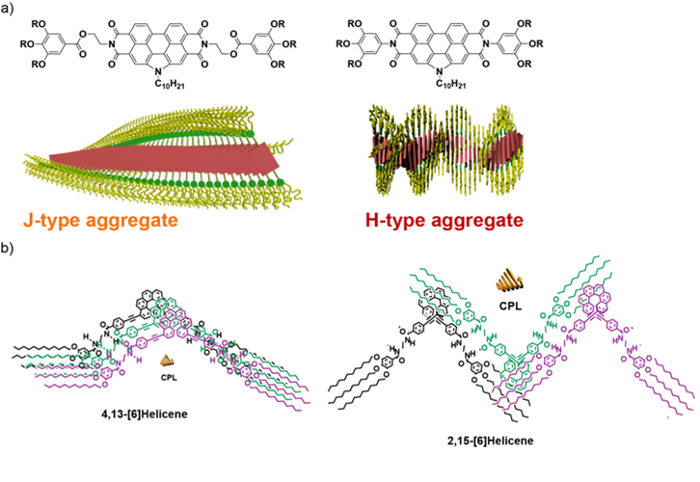
Herein, we will see recent results of the achievements reached in our research group about the formation of chiral supramolecular the supramolecular polymers. Thus, the strong influence of the side lateral groups in the supramolecular polymerization mechanism and chiroptical features of N-annulated perylenebisimides (Figure 1a), as well as the relevant role that the substitution pattern in [6]helicenes plays in the mutual orientation of the monomeric units in the aggregated state will be presented (Figure 1b).
References
- M. A. Martínez, A. Doncel-Giménez, J. Cerdá, J. Calbo, R. Rodríguez, J. Aragó, J. Crassous, E. Ortí, L. Sánchez, J. Am. Chem. Soc. 2021, 143, 13281−13291.
- R. Rodríguez, C. Naranjo, A. Kumar, P. Matozzo, T. K. Das, Q. Zhu, N. Vanthuyne, R. Gómez, R. Naaman, L. Sánchez, J. Crassous, J. Am. Chem. Soc. 2022, 144, 7709−7719.
