Research project
Supramolecular and Biomaterials Chemistry
Alexander Kros studies supramolecular systems in a biological environment. The unifying theme between the projects in my lab is specific molecular recognition, i.e. the intermolecular interaction between complementary molecules with high affinity and selectivity. Studying, imitating and dissecting processes from Nature and applying the underlying principles in model systems to mimic these processes or to design new functionalities is what drives our research. Examples are our efficient model system for membrane fusion, new drug delivery tools as well as designer biomaterials to obtain blood vessel networks for tissue engineering. Furthermore a novel allergy vaccine platform is currently being developed based on peptide amphiphile assembly.
- Contact
- Alexander Kros
Membrane fusion
Inspired by the natural membrane fusion machinery, the aim of this research line is to design a synthetic analogue in order to:
1) Understand the process of the peptide-controlled fusion of two membranes at the atomic, molecular and mesoscopic level.
2) Developing a new generic method for the controlled delivery of any (bio)molecule directly into the cytoplasm of a cell thereby omitting endocytotic pathways. This new paradigm opens up many new research applications in the fields of functional proteomics, genomics and siRNA-technology.
Studying, imitating and dissecting processes from Nature and applying the underlying principles has been highly successful approach for many years and opened up new lines of research and applications which were previously unimagineable. Examples are the aptamer- and antibody technology. Here we use this “learning-from-Nature” approach to design synthetic analogues of the membrane fusion machinery to create new functions and/or applications which are currently non-existent.
Single gold nanorods in live cells
Single-molecule fluorescence and force spectroscopy provide novel insight into the dynamics and interactions of biomolecules. Yet, current in vitro experiments are not always relevant to complex and variable cell conditions. However, tracking and manipulating single molecules inside a cell is still not possible. Together with Biophysics and Biology we will use modified gold nanorods (GNR) as a solution to this problem. In this project we will probe specific biomolecules inside a cell. Our contribution is to design GNR that specifically bind to a specific single protein in the cytoplasm. Furthermore we develop routes to delivery GNR into cells.
Supramolecular peptide amphiphiles as a novel allergy vaccine platform
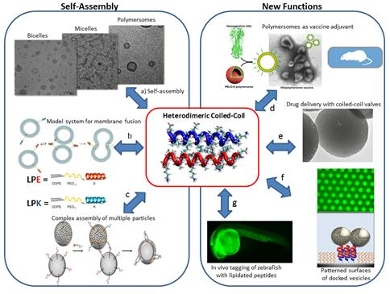
Peptide amphiphiles have come into the spotlight for supramolecular chemists in recent years. Conjugation of a peptide to a hydrophobic anchor strongly affects the supramolecular assembly and various morphologies have been reported. Currently, biomedical applications of peptide amphiphile-based materials are being explored. Together with HAL Allergy and the AMC, we aim to develop a new delivery platform for allergy vaccines with a high efficacy and a high safety profile. We will use peptide amphiphile nanoparticles based on coiled coil (CC) motifs as a delivery platform for allergy vaccines and study systematically the effect of size, shape and chemical composition on their efficacy.
Designer blood vessel networks for tissue engineering
One of the main hurdles in the engineering of artificial human tissues is the inability to recapitulate the intricate vascular networks that are present in normal tissues. The aim of my proposal is to control vascular network formation in engineered tissues through the localized and inducible release of a vascular guidance factor. Currently, methods to induce blood vessel growth in engineered tissues lead to the formation of random, disorganized and often dysfunctional vessel networks. Controlling the directional growth of blood vessels would allow the design of networks that mirror the normal tissue architecture. We develop an innovative and biocompatible material based on mesoporous silica nanoparticles (MSNs) that allows for spatial and temporal control over drug release that control vascular growth. The outcome will advance the field of vascular tissue engineering and will open a new application for mesoporous silica nanoparticles.
Drug delivery systems
Site-specific stimuli delivery systems are attractive materials as drug delivery system (DDS) for the treatment of cancer disease in which the ultimate goal is the release of significant amount of drug in selected areas without damaging surrounding healthy cells. Mesoporous silica nanoparticles (MSNs) are actively studied materials for drug delivery because of their fascinating properties. Recently we studied a new stimuli-responsive material based on silica mesoporous nanoparticles capped with rotaxane valves and a folic acid head group. The nanovalve system, so-called FAMSNs, consists of a monolayer of mechanically interlocked molecules in the form of the rotaxane composed of: a linear stalk anchoring the rotaxane on the surface, a gating ring which encircles it and lock the cargo in the mesopores, and a stopper located at the end of the linker connected to it with a cleavable bond. Herein, the so-called rotaxane nanovalves are activated by esterase enzymes while the targeting moiety folic acid is an integral part of the valve system. FAMSNs release their cargo upon a biological stimulus (i. e. esterase) and no leakage was observed in the absence of this enzyme. Thus this system prevents significantly severe side effects due to unspecific release of drug. The delivery of the anticancer drug led to the detected pharmacological effects as a significant concentration of compound was released in the cytoplasm of the cells.
Key publications
- Zope, H., C.B. Quer, P.H.H. Bomans, N.A.J.M. Sommerdijk, A. Kros, W. Jiskoot, "Peptide Amphiphile Nanoparticles Enhance the Immune Response Against a CpG-Adjuvanted Influenza Antigen", Advanced Healthcare Materials, vol. 3, no. 3, pp. 343-348, Mar, 2014. DOI: 10.1002/Adhm.201300247
- Zope, H.R., F. Versluis, A. Ordas, J. Voskuhl, H.P. Spaink, A. Kros, "In Vitro and In Vivo Supramolecular Modification of Biomembranes Using a Lipidated Coiled-Coil Motif", Angewandte Chemie-International Edition, vol. 52, no. 52, pp. 14247-14251, Dec 23, 2013. DOI: 10.1002/Anie.201306033
- Versluis, F., J. Voskuhl, B. van Kolck, H. Zope, M. Bremmer, T. Albregtse, A. Kros, "In Situ Modification of Plain Liposomes with Lipidated Coiled Coil Forming Peptides Induces Membrane Fusion", Journal of the American Chemical Society, vol. 135, no. 21, pp. 8057-8062, May 29, 2013. DOI: 10.1021/Ja4031227
- Porta, F., G.E.M. Lamers, J. Morrhayim, A. Chatzopoulou, M. Schaaf, H. den Dulk, C. Backendorf, J.I. Zink, et al., "Folic Acid-Modified Mesoporous Silica Nanoparticles for Cellular and Nuclear Targeted Drug Delivery", Advanced Healthcare Materials, vol. 2, no. 2, pp. 281-286, Feb, 2013. DOI: 10.1002/Adhm.201200176
- Peng, K., I. Tomatsu, B. van den Broek, C. Cui, A.V. Korobko, J. van Noort, A.H. Meijer, H.P. Spaink, et al., "Dextran based photodegradable hydrogels formed via a Michael addition", Soft Matter, vol. 7, no. 10, pp. 4881-4887, 2011.
