Research project
Operando research in electrochemistry
The central theme of Rik Mom’s research group is to identify what the electrode-electrolyte interface looks like when electrocatalysis is taking place. Using specialized forms of Raman and X-ray spectroscopy, we study the chemical state and bonding environment of the electrode surface and near-surface electrolyte. From the correlation of this chemical structure with the catalytic activity and electrode stability, we want to learn which structural features need to be controlled in order to create efficient and durable electrocatalytic processes. Our current research is focused on fuel cell and electrolyzer catalysis.
- Duration
- 2020
- Contact
- Rik Mom
In the future economy, the storage of electrical energy in chemical energy carriers such as hydrogen, ammonia and hydrocarbons will take a central place. Energy is transferred to or from these carrier molecules in electrocatalytic reactions in the few atomic layers around the electrode-electrolyte interface. The structure of these few atomic layers largely determines the performance of the overall process, i.e. its efficiency, selectivity and durability. Therefore, it is of great interest to study what the interface structure looks like, and how we can control it to our advantage.
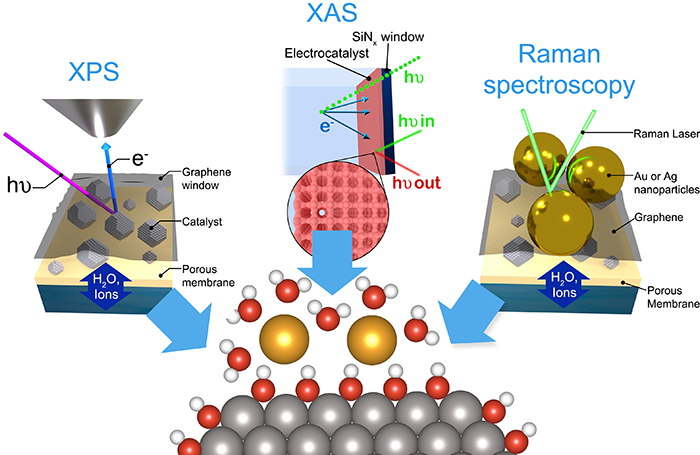
The key challenge is to specifically probe the electrode-electrolyte interface, and not the bulk electrode/electrolyte around it. To achieve this, we modify techniques from the traditional surface science toolbox to make them compatible with electrochemistry. For example, we use ultrathin graphene or silicon nitride windows to separate the vacuum in the measurement chamber from the electrolyte (see figure). Currently, we are developing new strategies in X-ray photoelectron spectroscopy (XPS), nanoparticle-enhanced Raman spectroscopy and X-ray absorption spectroscopy (XAS), often in collaboration with research groups at synchrotrons.
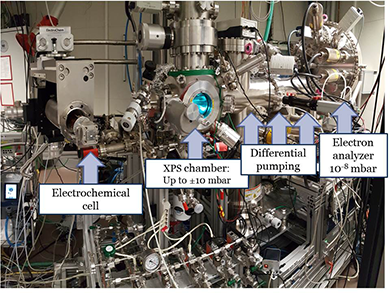
We currently study fuel cell and electrolyzer catalysis. In fuel cells, the oxygen-reducing cathode is the bottleneck for both the efficiency and device lifetime. Using model systems, we investigate the atomic origins of this bottleneck. Similarly, the oxygen-evolving anode is the limiting factor in electrolyzers. Requiring high potentials, the conditions of the oxygen evolution reaction are so corrosive that even the state-of-the-art electrocatalysts slowly dissolve during operation. In search for electrolyzer design criteria that maximize oxygen evolution and minimize electrode dissolution, we investigate the fundamental processes and active sites of the dissolution reaction, and their relation to the oxygen evolution catalysis.
