Publication
Fast Oxygen Reduction Catalyzed by a Copper(II) Tris(2‐pyridylmethyl)amine Complex through a Stepwise Mechanism
The mechanism of the electrochemical reduction of dioxygen by a mononuclear pyridylalkylamine copper complex was investigated (see picture). It was shown that in neutral aqueous solution dioxygen undergoes stepwise reduction, wherein hydrogen peroxide plays a key role. The rate constants determined for this electrocatalytic reaction are among the highest reported for molecular oxygen‐reduction catalysts.
- Author
- Langerman M., Hetterscheid D.G.H.
- Date
- 24 July 2019
- Links
- DOI link
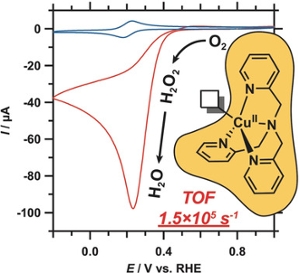
Abstract
Catalytic pathways for the reduction of dioxygen can either lead to the formation of water or peroxide as the reaction product. We demonstrate that the electrocatalytic reduction of O2 by the pyridylalkylamine copper complex [Cu(tmpa)(L)]2+ in a neutral aqueous solution follows a stepwise 4 e−/4 H+ pathway, in which H2O2 is formed as a detectable intermediate and subsequently reduced to H2O in two separate catalytic reactions. These homogeneous catalytic reactions are shown to be first order in catalyst. Coordination of O2 to CuI was found to be the rate‐determining step in the formation of the peroxide intermediate. Furthermore, electrochemical studies of the reaction kinetics revealed a high turnover frequency of 1.5×105 s−1, the highest reported for any molecular copper catalyst.
