
How cells talk by pulling on a fibre network
Mechanics play a larger role in blood vessel formation, and other developmental biology, than previously thought. Cells appear to respond to mechanical signals, such as pressure. Through the extracellular matrix, a network of fibrous proteins, cells can supposedly exchange those mechanical signals over long distances. To better understand how cells and matrix influence each other, researchers from the Leiden Mathematical Institute (MI) and the Institute of Biology Leiden (IBL) have developed a simulation model.
The formation of small blood vessels plays a role in things such as wound healing and tumour growth. The cells that will form these new blood vessels must be able to find each other over relatively long distances. New research suggests that cells exchange mechanical signals for this purpose. The extracellular matrix plays a role here.
A research group of Leiden mathematicians and biologists led by professor Roeland Merks introduces a new computer model to calculate the interaction between cells and the extracellular matrix. Their findings are published in the Biophysical Journal.
A kind of glue between cells
‘The extracellular matrix is a kind of glue between cells that consists of elongated, fibre-like proteins secreted by the cells,’ says Merks. This fibre network is found in tissues of animals and humans. But similar fibre networks are also found in the cell walls of plant cells, where they are partly composed of cellulose. It gives structure and strength. The network can also transmit mechanical signals as cells pull or push on fibres, causing a change further down the matrix. Other cells react to this deformation, which tells them which way to go in order to form blood vessels together, for example.
To better understand this process, the researchers developed a simulation model. ‘We have been working with the so-called cellular Potts model for some time,’ says Merks. This 30-year-old model describes the movement and behaviour of cells and cell deformation by external forces. ‘What was missing was a good representation of the extracellular matrix, although we know it is important.’
Bringing together chemistry and maths
To make the picture more complete, mathematician Bente Hilde Bakker combined the Potts model with a computational method from chemistry that can describe the fibres and interconnections of the extracellular matrix.
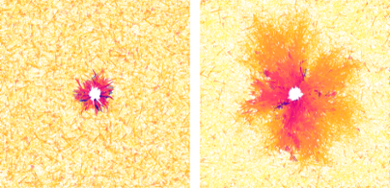
Bringing the two together was not easy. ‘The solution was inspired by what we see in real cells,’ says biologist Erika Tsingos, who tested the new combined model and compared it with experiments. ‘Cells make contact with the fibre network via proteins on their membrane. You can think of it as little hands that grasp the fibres and push and pull on them.’ They mimic this by digitally linking cell membranes and fibres at points where they overlap. The fibre model then registers a force at this particular point as the cell contracts. That force spreads through the network via the connections.
Better understanding blood vessel formation
Using this new model, one of the things Tsingos simulated was how the number of interconnections between fibres determine how the network reacts when a cell contracts. With many interconnections, the matrix was found to change shape down to a few cell lengths away. ‘That is consistent with the long-range effects we see in experiments with real cells,’ says Tsingos. ‘So our simulations seem to match biological systems.’
‘We have demonstrated that the technique works,’ says Merks. ‘The next step is to use the new computer model to better understand processes involving the extracellular matrix, such as blood vessel formation.’

The publication Hybrid cellular Potts and bead-spring modeling of cells in fibrous extracellular matrix appeared on the cover of the Biophysics Journal.
Images: Leiden University
Text: Dorine Schenk
