
Cathodic corrosion: devastating but predictable
An indian stepwell on a nanoscale. That is what postdoc Nakkiran Arulmozhi calls the pattern he saw when he corroded a special kind of platinum crystal. The unique images show the destructiveness of the process, but also show how predictable it is.

Corrosion can take place in different ways. Anodic corrosion, for example, is known as rust on your bicycle. The surface oxidises and the metal oxide formed may dissolve if the conditions are right. ‘At first, we thought that this would also happen with the real-world platinum electrodes,’ says Arulmozhi. Hitachi High-Tech Corporation, a Japanese company, asked Arulmozhi's supervisor Marc Koper, Professor of Catalysis and Surface Chemistry, to investigate the wear and tear of the electrodes in the hope that they could improve their lifetime.
Unexpected twist
The researchers soon discovered that something else was going on and published their findings in the journal PNAS. ‘It seems very likely that this is not anodic, but cathodic corrosion,’ says Koper. In this process, a metal is reduced, creating a metal hydride. ‘You would think that this is not possible at all, because a metal is already completely reduced. But under cathodic conditions, in other words at a negative voltage, platinum does corrode.’
The compounds that arise from cathodic corrosion are extremely unstable, so you cannot measure them directly. ‘We have to assume that they are formed and react with a water molecule within a very short time, causing them to oxidise again to platinum,' says Koper. 'What we can see, however, is that the structure of the material changes.’
Not randomly
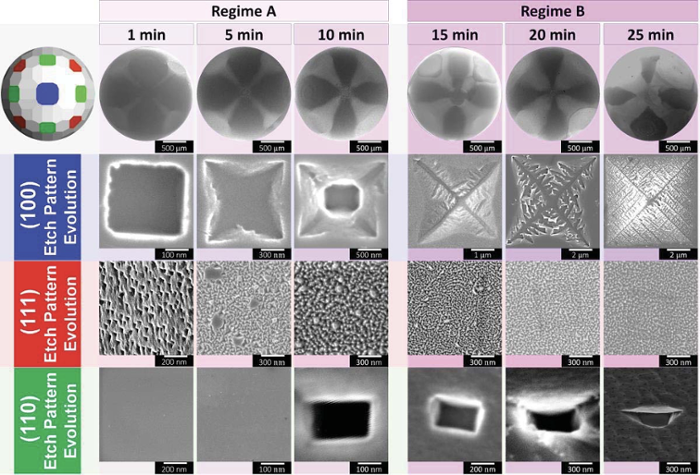
Arulmozhi visualised the process by corroding specially designed platinum crystals in a controlled way. A metal surface normally consists of a jumble of so-called facets. In each facet, the atoms are arranged in a specific way. Arulmozhi made the crystals in such a way that he knew exactly where each facet is located and how the atomic structure is constructed.
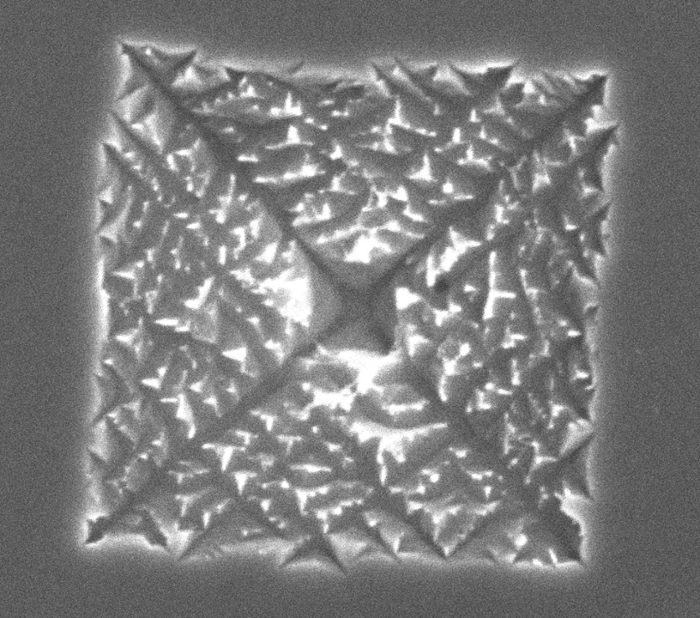
‘I saw that the wear process of the platinum differs per facet,’ says Arulmozhi. On the images, you can see how the green-coloured facet, Pt(110), hardly corrodes, while the blue-coloured surface, Pt(100), undergoes a process that the researchers call fractal etching. ‘The wear and tear starts in the form of a square. Slowly this changes into an inverted pyramid, in which eventually a beautiful fractal with various branches is created. They remind me of an Indian stepwell, but on a nanoscale.’
Wanted?
‘We never expected this process to be so orderly,’ says Koper. 'It makes cathodic corrosion predictable and hopefully we can make clever use of that, for example by designing platinum electrodes with only atomic structures that do not or hardly corrode.’
In other cases, cathodic corrosion is a desirable factor. ‘You can make nanoparticles with them,’ says Arulmozhi. ‘These are created when a metal particle breaks loose from the surface through corrosion and binds to another metal particle in the solution. In that case you want a material of facets that wear out easily, such as Pt(100).’
Publication
Nakkiran Arulmozhi, Thomas J. P. Hersbach & Marc T. M. Koper, Nanoscale morphological evolution of monocrystalline Pt surfaces during cathodic corrosion (PNAS, 7 December 2020)
Text: Michelle Wijma
