Lecture
LIC Lecture + drinks
- Date
- Tuesday 6 June 2023
- Time
- Location
-
Gorlaeus Building
Einsteinweg 55
2333 CC Leiden - Room
- EM1.09
With this event we celebrate the Marie Skłodowska-Curie postdoctoral fellowship of Irene Regeni and the honored European Doctoral Network ‘GLYCO-N’ in which Marta Artola and Hermen Overkleeft participate.
During the lecture Irene and Marta will present their research projects.
Programme
16:00 - 17:00 Introduction, Lectures (EM1.09)
17:00 - 18:00 Drinks (Atrium ground floor)
Irene Regeni - Ruthenium Peptide Bioconjugates for Photoactivated Chemotherapy
Biography
Irene Regeni studied chemistry at Trieste University (Italy) and obtained her master degree with Prof. Iengo on a joint project with Prof. Severin (EPFL, Lausanne). She did her PhD in Germany, at the TU Dortmund University, in the group of Prof. Guido Clever, for which she was awarded a “Kekule’ fellowship” from the Fonds der Chemischen Industrie and a Honorable Mention from the IUPAC-Solvay International Award for Young Chemists for the best PhD theses. The general focus of her PhD research was to implement the functionalities of commonly-known organic dyes into self-assembled Pd(II)-coordination cages to develop 3D nanoobjects interacting with visible light. In 2022, she joined the group of Prof. Bonnet at the Leiden Institute of Chemistry as a postdoctoral researcher. For this position, she was awarded two personal grants, a Walter Benjamin Program from the DFG (Deutsche Forschung Gemeinschaft) as well as a Marie Skłodowska-Curie Actions Postdoctoral Fellowship. Her current research focuses on developing new Ru(II)-polypyridyl peptide bioconjugates for photoactivated chemotherapy.
Abstract
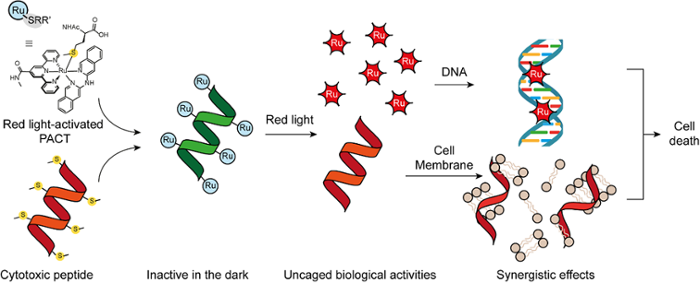
One of the most severe limitations of current anticancer chemotherapy are the serious side effects caused by toxic drugs affecting not only tumors but also healthy organs. Local activation of drugs by light irradiation of the tumor is a promising approach to control where the toxicity is delivered. Metal complexes are well suited for photoactivated chemotherapy, but their activation wavelength is often too low to afford high tissue penetration of light; also, their ability to enter cancer cells is often controlled by lipophilicity tuning, which is unselective; finally, their phototoxicity often relies on oxygen-dependent mechanisms, while many tumor tissues show low dioxygen concentrations.
The aim of this MSCA project is to develop new metallodrugs that are activated by red or near-infrared light, enter cells by controlled mechanisms, and deliver strong phototoxicity to cancer cells also under low oxygen conditions. The design is based on connecting multiple Ru(II) metal complexes to a biologically active antitumoral peptide. Both components will cage each other in the dark, thus affording low toxicity; while red light-induced cleavage of the ruthenium-thioether bonds will release two bioactive components, which will kill cancer cells.
Marta Artola - GLYCOprotein N-glycosylation from non-life to eukaryotes
Biography
Marta Artola performed her Ph.D. studies at Universidad Complutense de Madrid (Spain) where she worked on the validation of FtsZ protein as a new antibacterial target under the supervision of Prof. Maria Luz Lopez Rodriguez. In 2013 she was awarded with the young researcher JANSSEN-CILAG prize from the Spanish Society of Medicinal Chemistry (SEQT) and in 2014 she received her Ph.D. in Organic Chemistry with Cum Laude. During that period she visited Prof. Sieber’s Lab at the Technische Universität München (Germany) to investigate the antibacterial properties of her compounds, and later she joined Prof. Baran’s group at The Scripps Research Institute in San Diego (U.S.A) where she worked on the total synthesis of Ingenol analogues. In 2015, she moved to Leiden University and joined the Bio-organic Synthesis team as a postdoctoral researcher. During her postdoc she developed conformational inhibitors and activity-based probes (ABPs) for diverse glycosidases. In 2019, Marta was appointed as assistant professor at the Medical Biochemistry Department where she started her independent career combining organic chemistry, chemical biology and medical biochemistry. She currently supervises 6 PhD students and together they develop inhibitor libraries, protein degraders, ABPs and enzymatic substrates for glycoprocessing enzymes and RNA pseudoknots with the final goal of finding solutions for unmet medical needs in terms of target validation, diagnostics as well as drug discovery.
Abstract
The GLYCO-N is a new European doctoral network that brings together a diverse group of world-leading experts in microbiology, (bio)organic chemistry, structural biology, and chemical biology. GLYCO-N consortium will train 10 PhD candidates each with their own individual project to study and/or interfere with protein N-glycosylation from different angles.
Protein N-glycosylation, or the attachment of oligo- and polysaccharides at specific asparagine residues in a protein, is conserved throughout life, and is now also observed in viruses. In contrast to eukaryotes, whose well-studied N-glycosylation machineries are relatively simple, archaea, microalgae and bacteria utilize a wide variety of monosaccharides to create a wealth of structurally diverse N-glycans, and the same holds true for some recently discovered viruses. Because protein glycosylation occurs as a post-translational modification (so that genetics approaches are often not informative) the complexity and diversity in N-glycan structures are poorly understood in detail.
Therefore, understanding how and why N-glycosylation occur in archaea, microalgae and viruses will open up many possibilities ranging from drug discovery (antivirals) to biotechnology (glycoprotein and glycoprocessing enzyme engineering for materials and life sciences). To do so, microbiology on selected model species will identify N-glycoproteins and their processing enzymes, whose structures (N-glycoforms, glycosyltransferase (GT) and glycosidase (GH) families) will be resolved in molecular detail by structural biology (NMR, X-ray, EM) and bioinformatics. Synthetic organic chemistry guided by computational chemistry will then provide the tools to capture and study GT/GH mode of action with ABPs as well as lead-inhibitors for antivirals. Prof. Herman Overkleeft and Dr. Marta Artola will lead the (medicinal) chemistry and chemical biology team aiming to develop a synthetic toolbox of inhibitors and ABPs able to block and inform on protein N-glycosylation process.
