Research project
PKPD and disease modelling in therapy development for amyotrophic lateral sclerosis (ALS)
We aim to gain a better understanding of the natural disease progression of ALS in order to improve clinical trial design and analysis and to support drug discovery and clinical development of new drugs.
Amyotrophic lateral sclerosis (ALS) is progressive neurodegenerative disorder that causes death of motor neuron populations in the cerebral cortex, brain stem and spinal cord, leading to muscle weaknesses and eventually death (figure 1; left). The average life expectancy upon diagnosis is 3-5 years and with only one approved drug therapy, riluzole which prolongs life on average by 3 months, there is a high unmet medical need for new and improved therapies.
In this collaboration between the cluster Systems Pharmacology of the LACDR and the biotechnology company Treeway we aim to gain a better understanding of the natural disease progression of ALS in order to improve clinical trial design and analysis and to support drug discovery and clinical development of new drugs.
Pharmacokinetic (PK) and pharmacodynamic (PD) modelling and simulation is an important tool to investigate ADME (absorption, distribution, metabolism and excretion) properties of drugs and link plasma concentrations and drug exposure to intended and unintended (side-effects) responses in a variety of (patient)populations.
Development of a PK model for Treeway’s lead compound TW001 (oral edaravone) will help shed light on drug and formulation specific characteristics and the optimal dose and regimen for efficacy trials in the near future.

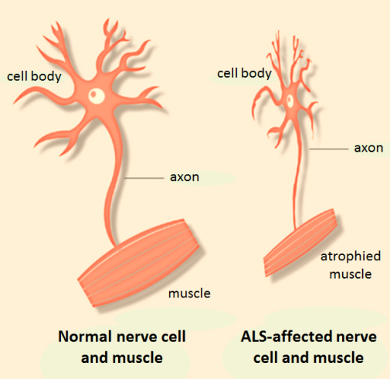
A difficult aspect of this complex disease is the large heterogeneity in natural disease progression, causing much uncertainty for patients with ALS and impeding the selection of a homogenous patient group for clinical trials (figure 1; right). Increased understanding of the patient characteristics and external factors influencing disease progression will be useful to predict disease progression on both the individual and population level, and improve patient selection and inclusion in future clinical trials.
In a separate part of this project, we aim to develop a pharmacometric disease progression model based on the ALS Functional Rating Scale (ALSFRS) and focus on the individual contribution of each sub-question of the ALSFRS questionnaire.
